Brain-Computer Interface Fundamentals: A Technical Deep Dive into Working Principles and Signal Acquisition
This article provides a comprehensive technical overview of Brain-Computer Interface (BCI) technology, with a focused examination of its core working principles and signal acquisition methodologies.
Brain-Computer Interface Fundamentals: A Technical Deep Dive into Working Principles and Signal Acquisition
Abstract
This article provides a comprehensive technical overview of Brain-Computer Interface (BCI) technology, with a focused examination of its core working principles and signal acquisition methodologies. Tailored for researchers, scientists, and drug development professionals, the content explores the foundational neuroscience behind BCIs, details the latest non-invasive, minimally invasive, and invasive signal acquisition technologies, and analyzes their respective performance benchmarks. It further addresses critical challenges in signal quality and system optimization, and offers a comparative validation of emerging technologies and their trajectories. The goal is to equip a technical audience with a clear understanding of the current state and future potential of BCI systems in biomedical research and clinical applications.
The Neuroscience and Core Architecture of Brain-Computer Interfaces
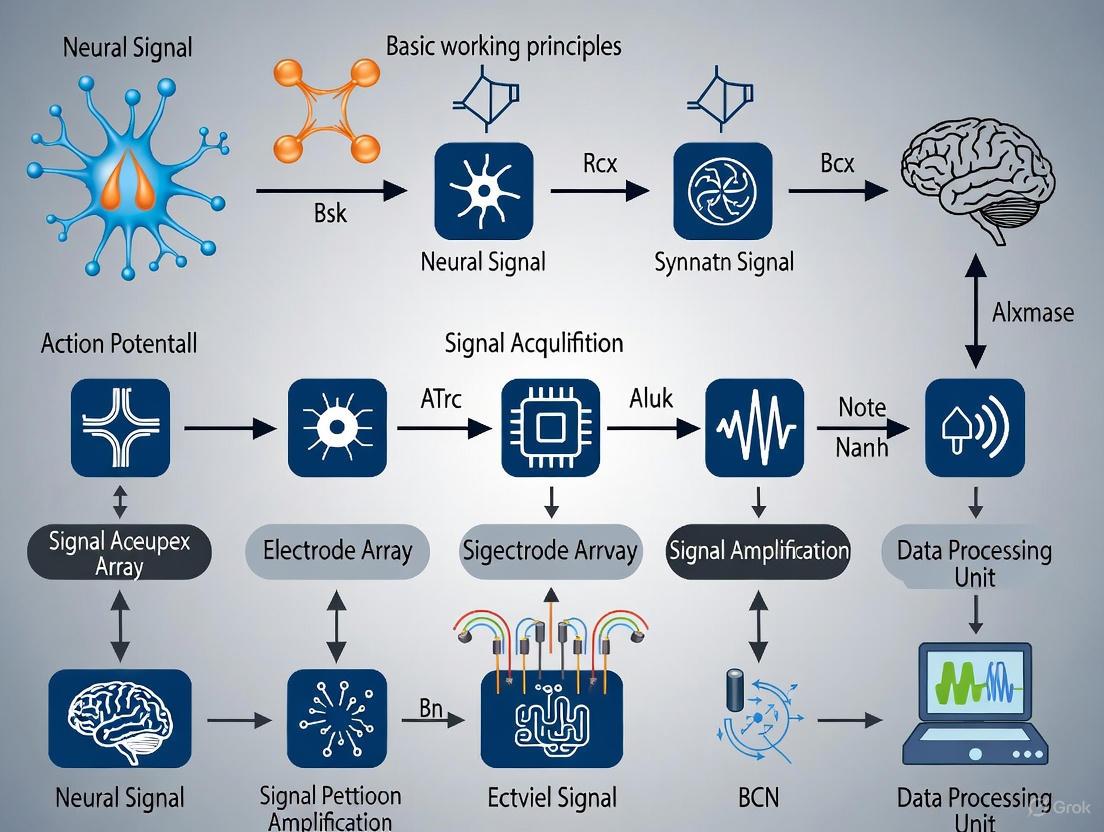
A Brain-Computer Interface (BCI) is a system that establishes a direct communication pathway between the human brain and an external device, translating thought into action independent of the brain's normal output pathways of peripheral nerves and muscles [1] [2]. This technology, which has evolved from a laboratory curiosity to a burgeoning neurotechnology industry as of 2025, enables users to control computers, robotic limbs, or communication aids through the decoding of their brain signals alone [1]. The core principle of any BCI is to acquire brain activity, decode the user's intent from this activity, and translate it into commands for an external device, creating a closed-loop system that can restore, replace, enhance, supplement, or improve natural central nervous system output [1] [3]. This in-depth technical guide outlines the fundamental working principles and signal acquisition research that underpin modern BCI systems, providing researchers and scientists with a foundational understanding of this transformative field.
Historical Context and Evolution
The conceptual and practical foundations of BCI technology were laid through pivotal milestones over the past century. The journey began in 1924 when Hans Berger recorded the first human electroencephalogram (EEG) from a young patient with cranial defects, using clay electrodes, thereby marking the inception of a scientific method for monitoring human brain activity [4]. Decades later, the term "brain-computer interface" was first formally articulated by Jacques Vidal in 1973, whose work is widely recognized as the foundational definition of a BCI as a device utilizing EEG signals for communication [4] [2]. At the inaugural international BCI conference in 1999, the technology was formally delineated as “a communication system that does not rely on the brain's normal output pathways of peripheral nerves and muscles” [4]. This definition was refined in 2012 to describe BCI as a “new non-muscular channel” for interaction, and again in 2021 with the introduction of the concept of a generalized BCI, characterized as “any system with direct interaction between a brain and an external device” [4].
Pioneering work has been instrumental in transitioning BCIs from concept to reality. In a notable 2014 self-experiment, neurologist Dr. Phil Kennedy paid to have electrodes implanted in his own brain after U.S. regulators halted his research. Despite post-operative complications that left him temporarily mute, his experiments proved that imagined speech could be captured and decoded from neural signals, providing a crucial proof of concept for giving voice to the voiceless [1]. As of mid-2025, BCI technology is in a phase of rapid translation from laboratory experiments to clinical trials, driven by numerous neurotech startups and research groups, standing roughly where gene therapies were in the 2010s—on the cusp of graduating from experimental status to regulated clinical use [1].
Core Working Principles of BCI Systems
The efficacy of a BCI system hinges on a structured, closed-loop pipeline that converts brain activity into functional outputs. This process involves four fundamental components that work in sequence: signal acquisition, processing (which includes decoding and translation), output, and feedback [1] [4] [2]. The following diagram illustrates this core BCI workflow and the interdependence of its components.
Diagram 1: The core closed-loop workflow of a BCI system.
Signal Acquisition
The process begins with signal acquisition, where electrodes or sensors pick up electrophysiological signals representing the brain's neurophysiological states [2]. The specific technologies and methodologies for acquisition are diverse and are discussed in detail in Section 4.
Processing and Decoding
The acquired raw signals are typically weak and contaminated with noise from various sources, including other biological signals (e.g., eye blinks, muscle activity) and environmental interference. Therefore, the processing stage involves multiple sub-stages [2] [5]:
- Preprocessing: The raw signals are filtered to remove artifacts, noise, and unwanted interferences, and are often amplified and digitized. This is a crucial step to ensure the data is clean and suitable for subsequent analysis [5].
- Feature Extraction: Critical electrophysiological features that define brain activities and encode the user's intent are extracted from the preprocessed signals. These features can be in the time-domain (e.g., amplitude or latency of event-related potentials like the P300) or frequency-domain (e.g., power spectra of sensorimotor rhythms) [2].
- Feature Classification and Translation: Using machine learning and classification methods (e.g., Support Vector Machines, Deep Learning), the system recognizes patterns in the extracted features that correspond to desired actions [4] [2]. The classified patterns are then translated into actual commands to operate an external device [2].
Output and Feedback
The translated commands are sent to an output device or application, which executes the user's intended action. This effector can be a robotic arm, a wheelchair, a computer cursor for letter selection, or a text-to-speech synthesizer [2] [3]. Finally, the feedback loop is critical for learning and accuracy. The user sees or hears the result of the action (e.g., a cursor moving or a letter being selected), which allows them to adjust their mental strategy accordingly, creating a closed-loop system that fosters adaptive learning and improved control [1] [4].
Signal Acquisition Technologies: A Dimensional Framework
The signal acquisition module bears the critical responsibility for the detection and recording of cerebral signals and is thus the most determinant component of BCI system performance [4]. A comprehensive understanding requires a dual-perspective analysis. A 2025 review by Sun et al. proposes a two-dimensional framework that synthesizes clinical (surgical) and engineering (detection) viewpoints, offering a holistic classification of BCI signal acquisition techniques [4] [6].
The Surgery Dimension: Invasiveness of Procedures
This dimension, classified from a clinician's perspective, refers to the invasiveness of the surgical procedure and its associated anatomical trauma [4]. The following table summarizes the three levels of this dimension.
Table 1: The Surgery Dimension of BCI Signal Acquisition
| Category | Definition | Surgical Trauma & Ethical Considerations | Clinical Oversight Requirements |
|---|---|---|---|
| Non-Invasive | Surgical actions for signal procurement do not induce anatomically discernible trauma [4]. | Minimal to none; lowest ethical intensity [4]. | Typically obviates the need for continuous clinical oversight [4]. |
| Minimally Invasive | Incurs anatomical trauma, but spares the brain tissue from impact [4]. | Moderate trauma; heightened ethical considerations compared to non-invasive [4]. | Necessitates the engagement of neurology or neurosurgery experts [4]. |
| Invasive | Causes anatomically discernible trauma at the micron scale or larger, specifically affecting the brain tissue [4]. | Highest degree of trauma; most intense ethical considerations [4]. | Virtually all methodologies require the direct involvement of experienced neurosurgeons [4]. |
The Detection Dimension: Operating Location of Sensors
This dimension, approached from an engineering perspective, is defined by the sensor's operating location and is directly linked to the theoretical upper limit of signal quality and biocompatibility risk [4]. The following table outlines the three levels of this dimension.
Table 2: The Detection Dimension of BCI Signal Acquisition
| Category | Definition & Sensor Location | Theoretical Signal Quality | Example Technologies |
|---|---|---|---|
| Non-Implantation | Signal is acquired through a sensor on the surface of the body [4]. | Lowest quality; akin to "listening to a chorus from outside the building" [4]. | Electroencephalography (EEG), Magnetoencephalography (MEG), functional Near-Infrared Spectroscopy (fNIRS) [3]. |
| Intervention | Sensor leverages naturally existing cavities (e.g., blood vessels) without harming original tissue integrity [4]. | Intermediate quality; a balance between signal fidelity and invasiveness [4]. | Stentrode (Synchron) [1]. |
| Implantation | Signal is collected from a sensor implanted within human tissue [4]. | Highest theoretical signal quality due to proximity to neural signal source [4]. | Microelectrode Arrays (e.g., Utah Array, Neuralink), Electrocorticography (ECoG) grids [1] [2]. |
The interplay between these two dimensions creates a comprehensive framework for classifying any BCI signal acquisition technology. The following diagram visualizes this two-dimensional framework, placing key technologies within the landscape defined by the surgery and detection dimensions.
Diagram 2: A two-dimensional framework for BCI signal acquisition technologies.
Modern BCI Platforms and Experimental Protocols
The theoretical principles of BCI are materializing through advanced platforms developed by both commercial entities and research institutions. These platforms serve as practical testbeds for the signal acquisition frameworks described above and provide tangible protocols for research and development.
Key Commercial and Research Platforms
As of 2025, several key players are leading the transition of BCI technology from lab to clinic through human trials [1]:
- Neuralink: Perhaps the most publicized BCI company, Neuralink is developing an ultra-high-bandwidth implantable chip with thousands of micro-electrodes threaded into the cortex by a robotic surgeon. The coin-sized implant aims to record from more neurons than any prior device. As of June 2025, the company reported that five individuals with severe paralysis are using Neuralink to control digital and physical devices with their thoughts [1].
- Synchron: In contrast to Neuralink’s open-brain surgery, Synchron’s Stentrode uses a minimally invasive, endovascular approach. The device is delivered via blood vessels (the jugular vein) and lodged in the motor cortex's draining vein, where it records brain signals through the vessel wall. Human trials have allowed participants with paralysis to control a computer for texting using thought alone [1].
- Precision Neuroscience: Co-founded by a Neuralink alumnus, Precision is developing an ultra-thin electrode array (Layer 7) designed to be placed on the cortical surface through a minimally invasive approach. Their "brain film" conforms to the brain's surface without piercing neural tissue, representing a compromise between non-invasive ease and invasive signal quality. In April 2025, Precision’s device received FDA 510(k) clearance for commercial use with implantation durations of up to 30 days [1].
- Paradromics: This company specializes in high-channel-count implants for ultra-fast data transmission. Its Connexus BCI uses a modular array with 421 electrodes and an integrated wireless transmitter. In June 2025, a University of Michigan team partnered with Paradromics to perform the first-in-human recording with the device during an epilepsy surgery. A full clinical trial focused on restoring speech is planned for late 2025 [1].
- Blackrock Neurotech: A long-time supplier of neural electrode arrays for academic research (notably the Utah array), Blackrock is now developing new electrode technology like Neuralace, a flexible lattice designed for less invasive cortical coverage and reduced scarring over time [1].
A Generalized Experimental Protocol for BCI Research
For researchers aiming to conduct BCI experiments, particularly in neurorehabilitation or communication, the following protocol outlines a generalized methodology derived from current practices and challenges [3] [5].
Participant Selection and Preparation:
- Cohort: Recruit participants based on the target application (e.g., patients with amyotrophic lateral sclerosis (ALS), spinal cord injury, or stroke for assistive technology; healthy controls for fundamental studies). Be mindful of the phenomenon of "BCI inefficiency," where 10-30% of users struggle to achieve control, often linked to individual variations in motor imagery capability [3].
- Ethical and Clinical Safeguards: For any protocol involving invasive or minimally invasive technologies, secure full regulatory (e.g., FDA) and institutional review board (IRB) approvals. Ensure the direct involvement of experienced neurosurgeons for invasive procedures [1] [4].
- Task Instruction: Train participants on the required mental strategy. This is typically either motor imagery (kinesthetic, focusing on the feeling of movement, is more effective than visual) or actual movement attempts. The quality of mental imagery significantly impacts the detectability of neural signals [3].
Signal Acquisition Setup:
- Technology Selection: Choose the acquisition technology based on the trade-off between required signal fidelity and acceptable invasiveness (see Section 4). Non-invasive EEG is common for initial studies, while invasive microelectrode arrays are used for high-bandwidth applications like speech decoding [1] [4].
- Calibration: Fit the sensor (e.g., EEG cap, implant) according to the manufacturer's and safety protocols. Record a baseline of brain activity.
Data Acquisition and Real-Time Processing:
- Paradigm Presentation: Present the user with a cue to perform the mental task (e.g., imagine moving their right hand). The timing and nature of these cues define the BCI paradigm (e.g., P300, Steady-State Visually Evoked Potential (SSVEP), sensorimotor rhythms) [7].
- Closed-Loop Operation: Run the core BCI workflow in real-time:
Output and Feedback Delivery:
- Effector Activation: Execute the command on the output device (e.g., move a robotic arm, select a letter on a screen, trigger functional electrical stimulation (FES) to move a paralyzed limb) [3].
- Feedback to User: Provide immediate, intuitive feedback (visual, auditory, or tactile) to the user about the system's action. This feedback is critical for the user to learn and adapt their mental strategy to improve control, thereby closing the loop [1] [3].
Post-Hoc Analysis and Validation:
- Performance Metrics: Quantify BCI performance using metrics such as information transfer rate (ITR), classification accuracy, and latency [2].
- Clinical Outcomes: For neurorehabilitation studies, assess functional improvements using standardized clinical scales for motor or communication function [3].
The Scientist's Toolkit: Key Research Reagents and Materials
Table 3: Essential Materials and Reagents for BCI Research
| Item | Function in BCI Research |
|---|---|
| Electrodes (Ag/AgCl, Gold, Microelectrodes) | Sensors for acquiring electrophysiological signals. Material and design (e.g., scalp, stent, cortical array) vary with the acquisition technology [1] [4]. |
| Electrolyte Gel | Ensures stable electrical conductivity and reduces impedance between the scalp and electrodes in non-invasive EEG systems [3]. |
| Signal Amplifier and Analog-to-Digital Converter (ADC) | Amplifies microvolt-level brain signals and converts them from analog to digital form for subsequent processing by a computer [2]. |
| Preprocessing Software (e.g., for ICA, Filtering) | Software tools for performing critical preprocessing steps like Independent Component Analysis (ICA) to remove ocular or muscular artifacts, and band-pass filtering to isolate relevant frequency bands [4] [3]. |
| Machine Learning Libraries (e.g., Scikit-learn, TensorFlow/PyTorch) | Libraries containing algorithms for feature extraction, classification, and translation, which are central to the decoding of the user's intent from brain signals [4] [2]. |
| Robotic Arm / Computer Cursor / FES Device | Common effector devices that serve as the output of the BCI system, providing tangible feedback and functional utility [2] [3]. |
The field of brain-computer interfaces has evolved from a foundational concept into a dynamic, interdisciplinary domain poised to revolutionize human-machine interaction, particularly in healthcare. The core working principle of a BCI rests on a closed-loop pipeline of signal acquisition, processing, output, and feedback. Modern research is increasingly guided by sophisticated frameworks that balance the critical trade-offs between signal fidelity and surgical invasiveness, as exemplified by the two-dimensional model of surgery and detection dimensions. As of 2025, the technology is in a pivotal translational phase, with numerous companies conducting human trials aimed at restoring motor and communication capabilities for people with severe paralysis. For researchers, the path forward involves not only refining signal acquisition and decoding algorithms for greater speed and accuracy but also rigorously addressing the accompanying ethical, clinical, and usability challenges to ensure these powerful technologies can be safely and effectively integrated into real-world applications.
Brain-Computer Interface (BCI) technology establishes a direct communication pathway between the human brain and external devices, bypassing conventional neuromuscular routes [8] [9]. This revolutionary technology translates raw neurophysiological data into actionable commands, enabling individuals to control computers, prosthetic limbs, and other devices through thought alone [10]. The fundamental signal flow within a BCI system follows a structured sequence: acquisition of brain signals, preprocessing to enhance quality, feature extraction and translation into commands, and finally, execution of those commands through an output device, often incorporating real-time feedback to create a closed-loop system [11]. This whitepaper provides a detailed, technical breakdown of this communication pathway, contextualized within contemporary research and advanced signal acquisition methodologies.
The Core BCI Signal Pathway
The following diagram illustrates the standardized, sequential flow of information in a closed-loop BCI system, from initial signal acquisition to the final device output and feedback.
Stage 1: Signal Acquisition
The first critical stage involves capturing electrical or metabolic signals generated by neural activity. The methodologies vary significantly in their invasiveness and signal resolution.
Table 1: Neural Signal Acquisition Methods in BCI Research
| Method | Principle | Interface Type | Key Applications | Signal Characteristics |
|---|---|---|---|---|
| Electroencephalography (EEG) [8] [12] | Measures electrical activity from the scalp surface. | Non-invasive | SSVEP paradigms [13], neurorehabilitation [11], cognitive monitoring. | Low signal-to-noise ratio, poor spatial resolution, high temporal resolution. |
| Electrocorticography (ECoG) [8] | Records electrical activity from the cortical surface. | Semi-invasive | Motor imagery, seizure focus localization, advanced communication. | Higher spatial and temporal resolution than EEG. |
| Intracortical Microelectrode Arrays [8] [12] | Records single-neuron or multi-unit activity from within brain tissue. | Invasive | High-performance prosthetics [10], complex device control. | Very high spatial and temporal resolution. |
| Functional Near-Infrared Spectroscopy (fNIRS) [8] | Measures hemodynamic responses (blood oxygenation). | Non-invasive | Cognitive state monitoring, stroke rehabilitation. | Lower temporal resolution, good for sustained states. |
Stage 2: Signal Preprocessing
Raw neural signals are inherently noisy and contaminated by artifacts (e.g., eye blinks, muscle movement, line noise) [11]. Preprocessing is essential to enhance the signal-to-noise ratio (SNR). Common techniques include:
- Filtering: Applying band-pass filters to isolate frequency bands of interest (e.g., Mu rhythm 8-12 Hz for motor imagery) and notch filters to remove power line interference [13].
- Artifact Removal: Utilizing algorithms like Independent Component Analysis (ICA) to identify and remove components of the signal not originating from brain activity [11].
Stage 3: Feature Extraction and Translation
This stage converts the preprocessed signals into meaningful control commands, heavily relying on advanced algorithms.
Feature Extraction
Distinctive patterns in the neural signals are identified. For EEG-based systems, this can include:
- Spectral Power: Quantifying power in specific frequency bands [11].
- Event-Related Potentials (ERPs): Detecting voltage fluctuations in response to specific stimuli, such as the P300 wave [13].
- Signal Complexity: Measuring features like spectral arc length for assessing movement smoothness [14].
Translation Algorithms
These algorithms map the extracted features to device commands. Machine Learning (ML) and Artificial Intelligence (AI) are central to modern BCI systems [11].
- Convolutional Neural Networks (CNNs): Effective for spatial and temporal pattern recognition in EEG data [13] [11].
- Support Vector Machines (SVMs): Used for classifying different mental states or movement intentions [11].
- Transfer Learning (TL): Reduces calibration time by adapting a pre-trained model to a new user, addressing the challenge of high inter-subject variability [11].
Table 2: Quantitative Performance of AI/ML Algorithms in BCI Systems
| Algorithm | Primary Function | Reported Performance | Key Advantage | Associated Challenge |
|---|---|---|---|---|
| Convolutional Neural Network (CNN) [13] [11] | SSVEP & Motor Imagery Classification | High accuracy in SSVEP signal recognition [13]. | Automatic feature learning from raw/poor signals. | Computationally intensive, requires large datasets. |
| Support Vector Machine (SVM) [11] | Mental State Classification | Used in cognitive assessment and AD/ADRD monitoring [11]. | Effective in high-dimensional spaces. | Performance depends on kernel selection. |
| Transfer Learning (TL) [11] | Cross-Subject/Cross-Session Adaptation | Reduced training time from weeks to under 4 hours with 95% accuracy [10]. | Mitigates calibration burden for new users. | Risk of negative transfer if data domains differ greatly. |
| Filter Bank CCA [13] | SSVEP Frequency Recognition | Enabled high ITRs for continuous control tasks like drone flight [14]. | Robustness against background EEG noise. | Specific to SSVEP paradigms. |
Experimental Protocol: SSVEP for Continuous Device Control
To illustrate the application of this pathway, the following workflow details a representative experiment for non-invasive, continuous control of a drone using Steady-State Visual Evoked Potentials (SSVEP) [14].
Detailed Methodology:
- Stimulus Presentation: The user is presented with visual stimuli flickering at distinct frequencies (e.g., 8.5 Hz, 10 Hz, 11.5 Hz) [13] [14]. In advanced setups, the drone's live video feed is embedded within these stimuli to provide a first-person perspective [14].
- Signal Acquisition & Preprocessing: EEG data is collected using a multi-channel headset. The raw data is band-pass filtered (e.g., 5-40 Hz) to isolate the frequencies relevant to the SSVEP response.
- Feature Extraction & Translation: The Filter Bank Canonical Correlation Analysis (FBCCA) algorithm is employed to identify the dominant SSVEP frequency in the user's EEG, which corresponds to the attended stimulus [14].
- Command Mapping & Output: The decoded frequency is mapped to a discrete command (e.g., "move forward," "turn left"). A novel control strategy then translates these discrete commands into smooth, continuous motions for the drone, enabling real-time navigation [14].
- Performance Metrics: The system's efficacy is evaluated using:
The Scientist's Toolkit: Essential Research Reagents & Materials
Table 3: Key Materials and Technologies for Advanced BCI Research
| Item / Technology | Function / Application | Research Context |
|---|---|---|
| High-Channel Count Acquisition Chip (e.g., SX-R128S4) [12] | High-throughput neural signal acquisition; enables recording from 128+ channels for high-resolution data. | Invasive BCI research for capturing detailed neural population activity. |
| Programmable Metasurface (STC) [13] | Provides visual stimulation for SSVEP and manipulates electromagnetic waves for secure wireless communication. | Used in secure BCI systems for harmonic-encrypted beamforming at the physical layer. |
| Utah Array / Intracortical Microelectrodes [10] [15] | Invasive neural interface for recording single-unit or multi-unit activity; provides the highest signal quality. | Foundation for high-fidelity BCI systems in clinical trials (e.g., BrainGate, Neuralink). |
| Dry EEG Electrodes [8] | Simplifies set-up for non-invasive BCIs by eliminating the need for conductive gel. | Consumer-grade and rapid-deployment BCI systems for usability outside the lab. |
| AI/ML Models (CNNs, TL) [13] [11] | Core software component for decoding neural signals and translating them into device commands. | Applied across all BCI types to improve accuracy, speed, and adaptability of systems. |
| Stentrode (Endovascular BCI) [10] [15] | Minimally invasive electrode array delivered via blood vessels; balances signal quality and safety. | Focus of clinical trials (e.g., by Synchron) for motor restoration without open-brain surgery. |
The BCI communication pathway is a sophisticated, multi-stage process that transforms neural signatures into functional control. Current research is focused on enhancing every stage of this pathway: developing higher-fidelity and less invasive acquisition hardware [12], creating more robust and adaptive AI-driven translation algorithms [11], and engineering closed-loop systems that provide meaningful feedback to the user [16]. The integration of advanced materials science, semiconductor technology, and artificial intelligence is pushing BCI technology from a primarily medical tool toward a future that may include seamless human-machine interaction across a wide spectrum of applications.
The human brain's operational currency is a complex symphony of bioelectrical signals, spanning multiple spatial and temporal scales. At the most fundamental level lies the action potential, a discrete all-or-nothing event facilitating long-distance communication along neuronal axons [17]. The coordinated synaptic and intrinsic transmembrane currents from neuronal populations collectively generate macroscopic field potentials, which include the local field potential (LFP), electrocorticogram (ECoG), and electroencephalogram (EEG) [18]. For brain-computer interface (BCI) technology, understanding this continuum from single-neuron spikes to population-level fields is paramount, as different BCI modalities tap into different levels of this signaling hierarchy. The efficacy of BCI systems is fundamentally contingent upon the precise acquisition and interpretation of these neural signals, which reflect the brain's intricate computational processes and provide the raw data for decoding intentional commands [6] [4].
Fundamental Mechanisms of Neural Signaling
Action Potentials: The Unit of Neural Communication
The action potential is a regenerative, self-propagating wave of electrical activity that travels along the axonal membrane of neurons [17]. According to the classical Hodgkin-Huxley model, this phenomenon arises from voltage- and time-dependent changes in the membrane's permeability to sodium (Na⁺) and potassium (K⁺) ions, leading to a characteristic waveform featuring a rapid depolarization followed by repolarization and a brief hyperpolarization [17]. This electrical impulse serves as a binary digital code for transmitting information over long distances within the nervous system with high fidelity, and its initiation in the axon initial segment follows an all-or-nothing principle [17].
Recent theoretical work suggests that action potential propagation may involve additional biophysical mechanisms beyond purely ionic conductance. Some models propose that the ion flow through Naᵥ channels generates a near-field quasi-static electric field (ephaptic field) in the extracellular space, which may facilitate excitation of nearby passive axons and enable zig-zag propagation patterns within axon bundles, potentially explaining the rapid transmission velocities observed in myelinated axons [19].
Table: Key Characteristics of Neural Action Potentials
| Property | Description | Functional Significance |
|---|---|---|
| All-or-None Principle | Once initiation threshold is reached, a full-amplitude spike occurs regardless of stimulus strength [17]. | Ensures faithful, high-fidelity transmission of information along axons. |
| Refractory Period | Brief period post-firing when neuron is resistant to generating another action potential [17]. | Enforces unidirectional propagation and limits maximum firing rate. |
| Ionic Basis | Primarily involves voltage-gated Na⁺ influx (depolarization) followed by K⁺ efflux (repolarization) [17]. | Generates characteristic spike waveform and enables regenerative propagation. |
| Propagation Velocity | Ranges from <1 m/s to >100 m/s; influenced by axon diameter and myelination [19]. | Determines timing and synchrony of information arrival in neural circuits. |
From Unitary Currents to Macroscopic Field Potentials
When action potentials reach presynaptic terminals, they trigger neurotransmitter release, which in turn generates postsynaptic potentials in target neurons. These transmembrane currents, along with other intrinsic membrane potential fluctuations, constitute the primary generators of the extracellular field potential [18]. The superposition of all ionic processes—from fast action potentials to slow synaptic and intrinsic currents—within a volume of brain tissue generates a measurable extracellular potential (Vₑ) at any given location [18].
The characteristics of the local field potential (LFP) waveform, including its amplitude and frequency content, depend on several factors: the magnitude and sign of individual current sources, their spatial density, the temporal coordination (synchrony) of these sources, and the passive electrical properties of the extracellular medium [18]. Synaptic activity is often the dominant contributor to LFP under physiological conditions, particularly excitatory postsynaptic currents (EPSCs) mediated by AMPA and NMDA receptors [18]. The influx of cations at the synapse creates an extracellular sink, which is electrically balanced by a distributed return current (source) along the neuronal membrane, forming a current dipole [18].
Table: Primary Contributors to Extracellular Field Potentials
| Source Type | Temporal Characteristics | Relative Contribution to LFP |
|---|---|---|
| Synaptic Currents | Relatively slow (milliseconds); excitatory and inhibitory [18]. | Often the most significant contributor in physiological conditions [18]. |
| Fast Na⁺ Action Potentials | Very fast (sub-millisecond) [18]. | Generates large-amplitude Vₑ deflections near soma; contributes to high-frequency components [18]. |
| Ca²⁺ Spikes | Slower than Na⁺ spikes [18]. | Can substantially shape extracellular field [18]. |
| Intrinsic Membrane Oscillations | Rhythmic, frequency-dependent [18]. | Contributes to specific frequency bands in the field potential [18]. |
| GABAₐ Receptor-Mediated Currents | Fast to medium kinetics [18]. | Can generate substantial transmembrane currents in depolarized neurons [18]. |
Signal Acquisition Methodologies in BCI Research
A Dimensional Framework for Neural Signal Acquisition
BCI signal acquisition technologies can be classified along two primary dimensions: the surgical invasiveness of the procedure and the operating location of the sensors [4]. This two-dimensional framework helps reconcile clinical considerations with engineering requirements, balancing signal fidelity against surgical risk and biocompatibility concerns [4].
The surgery dimension encompasses three categories of increasing invasiveness:
- Non-invasive: Procedures that cause no anatomically discernible trauma (e.g., EEG) [4].
- Minimal-invasive: Procedures causing anatomical trauma that spares brain tissue (e.g., endovascular stents) [4].
- Invasive: Procedures causing anatomically discernible trauma at the micron scale or larger to brain tissue (e.g., intracortical microelectrodes) [4].
The detection dimension classifies sensors based on their operational location:
- Non-implantation: Signals acquired through sensors on the body surface (e.g., scalp EEG) [4].
- Intervention: Sensors leveraging naturally existing cavities (e.g., blood vessels) without damaging tissue integrity [4].
- Implantation: Sensors placed within human tissue (e.g., intracortical arrays) [4].
BCI Signal Acquisition Framework Diagram
Comparative Analysis of BCI Recording Modalities
Different BCI recording modalities access neural signals at various spatial and temporal scales, creating a trade-off between signal resolution and invasiveness [18] [4].
Table: Comparison of BCI Signal Acquisition Technologies
| Modality | Spatial Resolution | Temporal Resolution | Primary Signals Captured | Key Advantages | Main Limitations |
|---|---|---|---|---|---|
| EEG (Electroencephalography) [18] | Low (integrated over ~10 cm² or more) | High (milliseconds) | Scalp-recorded macroscopic fields | Non-invasive, portable, low cost | Skull attenuates and spatially blurs signals, low signal-to-noise ratio [11] |
| ECoG (Electrocorticography) [18] | Intermediate (<5 mm² with grid electrodes) | High (milliseconds) | Cortical surface field potentials | Bypasses signal-distorting skull, higher spatial resolution than EEG | Requires craniotomy, limited to superficial cortical areas [18] |
| LFP (Local Field Potential) [18] | High (microns to millimeters) | High (milliseconds) | Local population activity near microelectrode | Direct brain access, records from deep structures, high information content | Invasive, risk of tissue damage, signal locality [18] |
| MEG (Magnetoencephalography) [18] | Intermediate (2-3 mm in principle) | High (milliseconds) | Magnetic fields from intracellular currents | Less dependent on conductivity of extracellular space than EEG | Expensive equipment, limited availability [18] |
| Intracortical Microelectrodes [4] | Very high (single neuron) | Very high (sub-millisecond) | Single/multi-unit activity & LFP | Gold standard for resolution, direct neural spiking | Highly invasive, biocompatibility challenges, signal stability [4] |
Experimental Protocols for Neural Signal Investigation
Protocol for Investigating Hierarchical Predictive Coding Signals
Predictive coding theory posits that the brain continuously generates prediction signals and prediction-error signals through hierarchical cortical interactions [20]. The following protocol outlines a method to disentangle these signals using EEG during an auditory local-global paradigm [20].
Objective: To identify hierarchical prediction and prediction-error signals and their spatio-spectral-temporal signatures in human EEG [20].
Experimental Design:
- Participants: 30 healthy adults with normal hearing [20].
- Stimuli: Three stimulus items: standard tone (x), deviant tone (y), and omission (o, no tone). Sequences consist of 2 or 3 items with three temporal structures: (xx/xxx), (xy/xxy), or (xo/xxo) [20].
- Block Design: 8 blocks of 144 trials each, with distinct configurations of sequence length and trial numbers to manipulate transition probabilities (TPx, TPy, TPo) and sequence probabilities (SPxx, SPxy, SPxo) [20].
- Counterbalancing: Each block delivered twice with reversed tone assignments (tone A as x/B as y, then B as x/A as y) to eliminate tone-specific effects [20].
Procedure:
- Participants listen to tone sequences while maintaining visual fixation and attention to sounds [20].
- EEG recorded using 64-channel system with appropriate sampling rate (≥1000 Hz) [20].
- Vigilance monitored throughout task [20].
Data Analysis:
- Preprocessing: Standard EEG preprocessing including filtering, artifact removal, and epoching [20].
- Tensor Decomposition: Use tensor-based decomposition method to extract prediction and prediction-error components from EEG responses [20].
- Model Fitting: Fit hierarchical predictive coding model to EEG data to quantitatively separate prediction and prediction-error signals [20].
- Spatio-Spectral-Temporal Analysis: Identify neural signatures in specific frequency bands (e.g., low beta, high beta, gamma) associated with prediction and prediction-error signals at different hierarchical levels [20].
Expected Outcomes: Identification of low-level prediction signals in high beta band representing tone-to-tone transitions, high-level prediction signals in low beta band representing sequence structure, and prediction-error signals in gamma band dependent on prior predictions [20].
Predictive Coding EEG Experiment Workflow
The Scientist's Toolkit: Essential Research Reagents and Materials
Table: Key Research Reagents and Materials for Neural Signal Investigation
| Item | Function/Application | Technical Notes |
|---|---|---|
| High-Density EEG System (64+ channels) | Recording macroscopic field potentials from scalp surface [18] [20]. | Enables source localization; requires conductive gel/paste; appropriate sampling rate ≥1000 Hz [20]. |
| Silicon-Based Polytrodes | High-density recording of LFP and single-unit activity in brain tissue [18]. | Multiple closely-spaced recording sites; minimal tissue damage; high spatial resolution [18]. |
| Voltage-Sensitive Dyes | Optical detection of membrane voltage changes in neuronal populations [18]. | Directly measures transmembrane voltage; limited by phototoxicity and signal-to-noise ratio [18]. |
| Flexible Subdural Grid Electrodes | ECoG recording directly from cortical surface [18]. | Platinum-iridium or stainless steel; spatial resolution <5 mm²; bypasses signal-distorting skull [18]. |
| Computational Modeling Software | Simulating neural dynamics and field potential generation [18]. | Implement Hodgkin-Huxley or predictive coding models; requires precise biophysical parameters [18] [20]. |
| Tensor Decomposition Algorithms | Separating interdependent neural signals (e.g., prediction vs. prediction-error) [20]. | Enables disentangling causally entangled signals in EEG/ECoG data [20]. |
The pathway from unitary action potentials to macroscopic field potentials represents a hierarchical cascade of neural information processing that forms the biological foundation for brain-computer interfaces. The biophysical principles governing action potential generation and propagation—including ionic conductance dynamics and potentially ephaptic coupling mechanisms—directly shape the extracellular signals that BCIs aim to capture and decode [18] [17] [19]. Understanding these fundamental mechanisms enables more sophisticated signal acquisition strategies that balance the trade-offs between invasiveness and resolution [4].
Future directions in BCI research will likely focus on closing the loop between neural signal decoding and adaptive stimulation, leveraging advances in artificial intelligence and machine learning to enhance signal classification and interpretation [11]. The integration of multi-scale recording approaches—combining information from single units, LFPs, and macroscopic fields—holds particular promise for developing more robust and naturalistic BCIs. As these technologies evolve, they will continue to be guided by our deepening understanding of how micro-scale neural events give rise to macro-scale field potentials that reflect the brain's computational states and intentional commands.
Brain-Computer Interface (BCI) technology establishes a direct communication pathway between the human brain and external devices, bypassing the body's normal neuromuscular output channels [21] [22]. The efficacy of any BCI system hinges on the effective implementation of its core neurophysiological paradigms—the specific mental tasks or stimulus presentation protocols designed to elicit distinguishable brain activity patterns [21]. This whitepaper provides an in-depth technical examination of three foundational BCI paradigms: Motor Imagery (MI), P300, and Steady-State Visual Evoked Potentials (SSVEP). Framed within the broader context of BCI basic working principles and signal acquisition research, this guide details the neural mechanisms, experimental protocols, and clinical applications of each paradigm, providing researchers with the essential knowledge for system design and implementation.
BCI Working Principles and Signal Acquisition Framework
A typical BCI system operates through a coordinated sequence of four stages: Signal Acquisition, Processing, Output, and Feedback, forming a closed-loop system [4] [23]. The initial acquisition stage is paramount, as it determines the quality and nature of the neural signals available for all subsequent decoding and control operations [4].
Signal acquisition technologies can be classified along two primary dimensions: the surgical invasiveness of the procedure and the operational location of the sensors [4]. The surgery dimension encompasses non-invasive (no anatomical trauma), minimally-invasive (trauma that spares brain tissue), and invasive (trauma affecting brain tissue) procedures. The detection dimension classifies technologies based on whether sensors operate via non-implantation (on the body surface), intervention (within natural body cavities like blood vessels), or implantation (within human tissue) [4]. As one progresses from non-invasive to invasive methods, the theoretical upper limit of signal quality increases, but this comes with heightened surgical risk, ethical considerations, and implementation complexity [4].
Table: Classification of BCI Signal Acquisition Technologies
| Surgery Dimension | Detection Dimension | Example Technologies | Key Characteristics |
|---|---|---|---|
| Non-Invasive | Non-Implantation | EEG, MEG, fMRI [21] [22] | Safe, low cost; lower spatial resolution and signal-to-noise ratio |
| Minimally-Invasive | Intervention | Endovascular Stent Electrodes [4] | Moderate signal quality; requires clinical specialists |
| Invasive | Implantation | Microelectrode Arrays, ECoG [21] [22] [4] | High signal fidelity; requires involvement of neurosurgeons |
The selection of an appropriate paradigm is deeply interdependent with the choice of signal acquisition method. Well-designed paradigms enhance the detectability and separability of brain signals within the constraints of the chosen acquisition technology [21] [23].
Motor Imagery (MI) Paradigm
Neural Basis and Principles
The Motor Imagery (MI) paradigm is based on the mental rehearsal of a motor act without any overt physical movement or external stimulus [23]. Kinesthetic imagination (feeling the movement) activates neural circuits in the primary motor cortex (M1), premotor cortex, and supplementary motor area that substantially overlap with those involved in actual motor execution [24] [23]. This mental process induces oscillatory changes in sensorimotor rhythms, specifically the mu (8-13 Hz) and beta (18-26 Hz) frequency bands [24]. The key phenomenon is Event-Related Desynchronization (ERD), a power decrease in these rhythms contralateral to the imagined limb, reflecting cortical activation [24]. Sometimes, an Event-Related Synchronization (ERS), a power increase, can be observed in ipsilateral regions [23].
Experimental Protocol and Design
A typical MI-BCI protocol for upper limb rehabilitation involves several key stages [24]:
- Participant Preparation and Setup: Participants sit comfortably in a chair, minimizing eye blinks and body movements. EEG is recorded using systems like the BioSemi ActiveTwo with 64 electrodes arranged in the international 10-20 montage [25]. Signals are referenced to the right mastoid and grounded to the left, sampled at 2048 Hz, and then down-sampled to 256 Hz for analysis. Artifact removal is performed using an infinite impulse response filter and wavelet-based neural networks [25].
- Task Design and Cue Presentation: The paradigm consists of multiple trials. Each trial begins with a fixation cross displayed on the screen for 2 seconds. A visual cue (e.g., an arrow or picture of a hand/foot) then instructs the participant to perform kinesthetic imagery of a specific movement (e.g., left hand, right hand, or foot) for a duration of 3-5 seconds [25] [24].
- Data Acquisition and Preprocessing: EEG data is recorded during the task performance. Standard preprocessing includes band-pass filtering (e.g., 1-50 Hz), common average re-referencing, and artifact removal for ocular, muscular, or other noise sources [25].
- Feature Extraction and Classification: Features are extracted from the mu and beta bands, often focusing on ERD/ERS patterns. Common spatial patterns (CSP) is a widely used algorithm for feature extraction. These features are then fed into classifiers like Linear Discriminant Analysis (LDA) or Support Vector Machines (SVM) to decode the intended movement [25].
- Feedback and Reinforcement: In closed-loop rehabilitation systems, the classifier's output is translated into a control signal for an external device, such as a functional electrical stimulation (FES) system or a robotic arm, providing the user with real-time feedback on their mental task performance [24].
Enhancements and Clinical Applications
A significant challenge in MI-BCI is "BCI inefficiency," where a portion of users cannot generate classifiable signals [25]. To address this, hybrid training approaches have been developed. For example, Somatosensory-Motor Imagery (SMI) combines MI with somatosensory attentional orientation (SAO) using tangible objects (e.g., a hard ball). This method has been shown to improve classification performance, particularly in poor performers, by engaging both motor and somatosensory cortices [25].
Another innovative approach is task-to-task transfer learning, which leverages the shared neural mechanisms between Motor Execution (ME), Motor Observation (MO), and MI. Training a classification model on the easier ME or MO tasks and then applying it to MI data can achieve similar accuracy while reducing user fatigue and calibration time, making the system more user-friendly [26].
In clinical practice, MI-BCI has shown significant promise in stroke rehabilitation. A randomized controlled trial demonstrated that stroke patients who received MI-BCI therapy combined with conventional rehabilitation showed greater improvement in upper extremity function (measured by Fugl-Meyer Assessment) and exhibited significant changes in cortical activation patterns on fMRI, compared to a control group receiving only conventional therapy [24]. The therapy induces beneficial functional activity-dependent plasticity, promoting motor recovery [24].
Table: Key Research Reagents and Materials for MI-BCI Research
| Item | Specification / Example | Primary Function in Research |
|---|---|---|
| EEG Acquisition System | BioSemi ActiveTwo, g.USBamp [25] [27] | Records scalp electrical activity with high temporal resolution. |
| EEG Electrodes/Cap | 64-channel Electro-Cap [25]; g.SAHARA dry electrodes [27] | Interfaces with the scalp for signal recording; dry electrodes offer easier setup. |
| Electrode Gel | Electrolytic gel | Ensures good electrical conductivity for wet electrode systems. |
| Stimulus Presentation Software | Unity, BCI2000 [28] [27] | Presents visual cues and controls experimental timing. |
| Feedback Devices | Robotic Arm, Functional Electrical Stimulation (FES) system [24] | Provides real-time, tangible feedback to user, reinforcing neural plasticity. |
| fMRI Scanner | 3T MRI Scanner [24] | Validates neural correlates of MI and measures therapy-induced plasticity. |
| Data Analysis Tools | MATLAB, EEGLAB, BCILAB | For signal preprocessing, feature extraction (CSP), and classification (LDA, SVM). |
P300 Paradigm
Neural Basis and Principles
The P300 is an event-related potential (ERP) component observed in the EEG approximately 250-500 ms after the presentation of a rare, task-relevant, or surprising stimulus [28]. Its amplitude is inversely related to the probability of the target stimulus, and it is considered a neural correlate of context updating or attention allocation [28]. In BCI systems, the P300 speller paradigm, first described by Farwell and Donchin, presents a matrix of characters to the user. The user focuses attention on a desired character (the target) while rows and columns of the matrix flash in a random sequence. Each time the row or column containing the target character flashes, it elicits a P300 response, allowing the system to identify the intended character [28].
Experimental Protocol and Design
The standard P300 speller experiment involves the following steps [28]:
- Stimulus Presentation: A 6x6 or similar matrix of characters is displayed. The stimulus presentation is characterized by the flash rate, which is the frequency at which the rows/columns intensify. Studies have shown that lower flash rates (e.g., 8-16 Hz) generally produce higher P300 amplitudes and better classification accuracy, though the optimal rate for information transfer rate may vary by user [28].
- Data Acquisition: EEG is typically recorded from 16 scalp electrodes (e.g., Fz, Cz, Pz, Oz). The signals are amplified, digitized (e.g., at 512 Hz), and band-pass filtered (e.g., 0.5-30 Hz) [28].
- Signal Processing and Classification: Epochs of EEG data (e.g., 0-800 ms post-stimulus) are extracted for each flash. A stepwise linear discriminant analysis (SWLDA) is commonly used for classification, where the system learns to distinguish between target flashes (which elicit a P300) and non-target flashes [28]. The character that receives the highest discriminant score when its corresponding row and column flash is selected as the output.
Table: Impact of Stimulus Parameters on P300 BCI Performance
| Parameter | Effect on P300 & Performance | Optimal Range / Notes |
|---|---|---|
| Flash Rate | Lower rates increase P300 amplitude but may slow communication speed [28]. | 8-16 Hz (user-dependent); optimal for characters/min varies. |
| Inter-Stimulus Interval (ISI) | Longer ISI (time between flash onsets) produces larger P300 and late negative slow wave amplitudes [28]. | 125-250 ms (for 4-8 Hz rates). |
| Number of Flash Repetitions | More repetitions increase classification accuracy but reduce selection speed. | Typically 10-15 repetitions per character selection. |
| Stimulus Type | Intensification of rows/columns is standard; other modalities (e.g., auditory) are also used. | Visual intensification is most common and effective. |
Steady-State Visual Evoked Potential (SSVEP) Paradigm
Neural Basis and Principles
The Steady-State Visual Evoked Potential (SSVEP) is an oscillatory brain response elicited in the visual cortex when a user gazes at a visual stimulus flickering at a constant frequency, typically between 3.5-75 Hz [29]. The resulting EEG signal shows peaks of activity at the fundamental frequency of the stimulus and its harmonics, effectively "entraining" the brain's rhythmic activity to the external stimulus [27] [29]. SSVEPs have a high signal-to-noise ratio and require minimal user training, making them suitable for high-information-transfer-rate BCIs [27].
Experimental Protocol and Design
A standard SSVEP-BCI setup involves [27]:
- Stimulus Design: Multiple visual targets are presented on a screen, each flickering at a distinct frequency. Frequencies are often chosen from prime numbers in the highly responsive range (e.g., 7, 11, 13, 17, 19, 23 Hz) to minimize interference from harmonics [27].
- Stimulation Methods:
- Single-Frequency Flicker: Each target flickers at a single frequency [27].
- Multi-Frequency Coding (Dual/Tri-Frequency): To increase the number of possible commands without requiring more frequencies, each stimulus can be coded with a unique combination of multiple frequencies (e.g., two or three). Methods for combining frequencies include frequency superposition (OR, ADD logic) and checkerboard patterns [27].
- Data Acquisition: EEG is recorded from occipital and parieto-occipital channels (e.g., PO3, POz, PO4, O1, Oz, O2) using a sampling rate of 512 Hz or higher. A 50 Hz/60 Hz notch filter is applied to remove line noise [27].
- Signal Processing and Classification: The canonical correlation analysis (CCA) method is a standard and efficient algorithm for detecting SSVEPs. CCA finds the linear correlation between the multi-channel EEG data and a set of reference signals at the stimulus frequencies and their harmonics, identifying the target frequency that maximizes the correlation [27].
Table: SSVEP Stimulation Paradigms and Their Characteristics
| Paradigm | Stimulation Method | Mechanism | Advantages & Applications |
|---|---|---|---|
| Single-Frequency | Simple flickering of a field or object [27]. | u = ½ sgn(sin(2πft)) + ½ | Simple to implement; suitable for systems with few commands. |
| Dual-Frequency (Superposition OR) | Two square waves superimposed with OR logic: S_OR,2 = u1 ∨ u2 [27]. | Stimulus is ON if either frequency signal is ON. | Increases the number of unique targets without increasing the base frequency pool. |
| Dual-Frequency (Superposition ADD) | Two square waves superimposed with ADD logic: S_ADD,2 = ½ u1 + ½ u2 [27]. | Brightness from two frequencies is summed. | Increases the number of unique targets without increasing the base frequency pool. |
| Checkerboard | Two frequencies delivered separately in alternating squares of an 8x8 checkerboard [27]. | Delivers two frequencies spatially separated within one stimulus. | Reduces perceptual interference between frequencies. |
The three core BCI paradigms each possess distinct characteristics that make them suitable for different applications and user groups.
Table: Comparative Summary of Key BCI Paradigms
| Feature | Motor Imagery (MI) | P300 | Steady-State VEP (SSVEP) |
|---|---|---|---|
| Neural Basis | Endogenous ERD/ERS of sensorimotor rhythms [23]. | Exogenous P300 event-related potential [28]. | Exogenous entrainment of visual cortex oscillations [29]. |
| Key Signal Features | Power decrease/increase in Mu/Beta bands (8-30 Hz) [24]. | Positive peak ~300-500 ms post-stimulus [28]. | Peaks at stimulus frequency and harmonics [27]. |
| User Task | Imagine body movement without executing it [23]. | Attend to rare target stimuli among frequent non-targets [28]. | Gaze at a flickering visual stimulus [27]. |
| Typical Accuracy | Varies widely; can be >80% in good performers, but ~50-70% in poor performers [25]. | Generally high (>90% with sufficient repetitions) [28]. | Generally high (>90%) [27]. |
| Information Transfer Rate | Low to Moderate | Moderate | High |
| User Training Required | Extensive training often needed [25] [26]. | Minimal training required [28]. | Minimal training required [27]. |
| Primary Clinical Application | Neurorehabilitation (e.g., stroke) [24]. | Communication for severely paralyzed (e.g., ALS) [28]. | Communication and environmental control. |
In summary, MI, P300, and SSVEP represent three pillars of non-invasive BCI technology. The choice of paradigm is a fundamental decision that directly impacts system performance, user experience, and clinical applicability. MI offers an intuitive, endogenous control strategy highly suited for motor rehabilitation. The P300 provides a reliable communication channel with minimal user training. SSVEP enables high-speed communication through exogenous visual entrainment. Future developments will likely focus on user-centered design, hybrid paradigms that combine the strengths of multiple approaches, and the integration of advanced AI to improve decoding performance, ultimately making BCI technology more robust and accessible for a wider range of applications [21].
The closed-loop brain-computer interface (BCI) represents a paradigm shift in human-computer interaction, establishing a direct bidirectional communication pathway between the brain and external devices. This integrated system architecture significantly enhances the efficacy, adaptability, and user-specificity of neurotechnology applications across medical, rehabilitative, and augmentative domains. By dynamically processing acquired neural signals and providing targeted feedback, closed-loop BCIs create an adaptive interaction cycle that enables real-time modulation of brain activity. This technical review comprehensively examines the core components, operational principles, and experimental methodologies underlying closed-loop BCI systems, with particular emphasis on their working principles and signal acquisition foundations. The synthesis of these elements creates a robust framework for developing next-generation neurotechnologies capable of restoring, replacing, and enhancing human cognitive and motor functions.
Brain-computer interface (BCI) technology has evolved from a scientific concept to a transformative tool that establishes a direct communication channel between the brain and external devices, bypassing conventional neuromuscular pathways [30]. A closed-loop BCI system represents the most advanced implementation of this technology, characterized by its bidirectional flow of information—reading neural signals and writing feedback stimuli in a continuous, adaptive cycle [30]. This self-regulating architecture stands in stark contrast to open-loop systems, which operate without incorporating user feedback to adjust their output [30].
The fundamental operational principle of closed-loop BCI relies on its four integrated components: signal acquisition, processing, output, and feedback [4]. These components work in concert to form a continuous loop that enables the system to dynamically adapt to the user's changing neural states and intentions. The system's ability to sense the user's physiological composition and deliver stimulation only when required represents a significant advancement in treatment modalities for neurological disorders, potentially reducing side effects and conserving power [30]. This integrated approach has profound implications for clinical applications, particularly for patients with complex nervous system diseases such as Alzheimer's, Parkinson's disease, dementia, and depression [30].
Core Components of a Closed-Loop BCI System
Signal Acquisition: The Input Gateway
Signal acquisition forms the critical first stage of any BCI system, responsible for detecting and recording cerebral signals with sufficient fidelity for subsequent processing and decoding [4]. The efficacy of the entire BCI system is predominantly contingent upon its signal acquisition module, which bears the fundamental responsibility for capturing neural activity [4]. Acquisition technologies can be classified through a two-dimensional framework encompassing both surgical invasiveness and sensor operating location [4].
Table 1: Classification of BCI Signal Acquisition Technologies
| Surgical Dimension | Detection Dimension | Example Technologies | Signal Quality | Clinical Risk |
|---|---|---|---|---|
| Non-invasive | Non-implantation | EEG, fNIRS, MEG [30] [8] | Low to Moderate | Minimal |
| Minimal-invasive | Intervention | Endovascular stents [4] | Moderate to High | Moderate |
| Invasive | Implantation | ECoG, Intracortical microelectrodes [30] [4] | High | Significant |
Electroencephalography (EEG) remains the most prevalent non-invasive acquisition method due to its portability, affordability, and temporal resolution [30]. EEG-based systems can be further categorized according to the nature of the recorded brain activity: "evoked" potentials (such as P300 and steady-state visually evoked potential - SSVEP) generated in response to external stimuli, and "spontaneous" activity (such as motor imagery - MI) generated volitionally by the user [30]. Invasive techniques, including electrocorticography (ECoG) and intracortical microelectrode arrays, capture signals directly from the brain's surface or cortical layers, providing superior spatial resolution and signal-to-noise ratio at the cost of requiring surgical implantation and associated clinical risks [30] [4].
Signal Processing: From Raw Data to Decoded Intent
The processing component transforms raw, often noisy neural signals into interpretable commands through a multi-stage algorithmic pipeline [31] [4]. This transformation involves preprocessing to enhance signal quality, feature extraction to identify discriminative neural patterns, and classification to decode the user's intended action [31].
Preprocessing techniques typically include filtering to isolate frequency bands of interest and artifact rejection methods to remove contamination from ocular, muscular, or environmental sources [31]. Subsequent feature extraction identifies salient characteristics in the neural signals, which may include time-domain amplitudes, frequency power distributions, or spatial activation patterns [31]. Finally, decoding algorithms translate these features into control commands using machine learning approaches such as linear discriminant analysis, support vector machines, or deep neural networks [31] [4].
The transition from offline analysis of recorded data to online, real-time processing represents a critical qualitative leap in BCI development [31]. While offline evaluation helps identify promising algorithms, online closed-loop testing remains the gold standard for validating BCI performance, as it accounts for the dynamic interaction between the user and the system [31].
Output Generation: Executing Commands
The output component translates the decoded neural commands into concrete actions executed through external devices [4]. This component serves as the effector mechanism of the BCI system, bridging the decoded neural intent with tangible outcomes in the user's environment.
Output devices span a diverse spectrum of applications, including:
- Neuroprosthetics: Robotic arms, grasping devices, and exoskeletons that restore motor function [30] [8]
- Communication aids: Spelling systems and speech synthesizers that enable expression for non-verbal individuals [8]
- Environmental controllers: Wheelchair navigation systems and smart home interfaces that increase autonomy [30]
- Functional stimulation systems: Devices that activate paralyzed muscles to restore movement [30]
The effectiveness of the output component depends critically on its precise synchronization with the decoded neural commands and its reliability in executing intended actions consistently across diverse usage contexts.
Feedback: Closing the Loop
Feedback constitutes the defining element of closed-loop BCI systems, completing the communication cycle by informing the user about the system's interpretation of their neural activity and the resulting outcome [30] [4]. This component enables real-time adjustment and learning by both the user and the system, creating a dynamic adaptive process that is fundamental to closed-loop operation.
Feedback can be provided through various sensory modalities:
- Visual feedback: Computer cursors, virtual avatars, or graphical representations [31]
- Auditory feedback: Tones, speech, or spatial sounds that convey information about performance [4]
- Tactile feedback: Vibrotactile or electrotactile stimulation that provides somatosensory information [8]
- Direct neural stimulation: Electrical, magnetic, or optogenetic stimulation that directly modulates neural pathways [30]
The feedback mechanism enables the crucial process of neuroplasticity, where both the user's brain and the BCI system adapt to each other over time, leading to improved performance and more efficient interaction [30]. This bidirectional adaptation represents the cornerstone of advanced closed-loop BCI systems, particularly those designed for therapeutic applications where the goal is to induce lasting neural changes [30].
Figure 1: Closed-Loop BCI System Architecture. This diagram illustrates the bidirectional information flow in a closed-loop BCI system, highlighting the continuous cycle between user neural activity and system feedback.
Experimental Protocols and Methodologies
Protocol for Evaluating Closed-Loop BCI Performance
Comprehensive evaluation of closed-loop BCI systems requires a multifaceted approach that assesses both technical performance and user experience metrics [31]. The following protocol outlines a standardized methodology for system validation:
1. Participant Preparation and Calibration
- Recruit participants representing the target user population (e.g., patients with specific neurological conditions or healthy controls)
- Apply signal acquisition devices according to established safety protocols
- Conduct an initial calibration session to personalize signal processing parameters
- Record 5-10 minutes of baseline neural activity for subsequent normalization
2. System Configuration
- Set sampling rates appropriate for the acquisition technology (e.g., 250-1000 Hz for EEG, 2000 Hz for ECoG)
- Configure filter settings: bandpass 0.5-40 Hz for movement-related potentials, 70-200 Hz for high-frequency activity
- Initialize decoding algorithm with pre-trained or participant-specific model
- Set feedback parameters (type, intensity, timing) based on experimental conditions
3. Experimental Tasks
- Implement standardized BCI paradigms:
- Motor imagery: Hand, foot, or tongue movement imagination
- Visual evoked potentials: P300 speller or SSVEP paradigms
- Cognitive tasks: Mental calculation, spatial navigation, or working memory
- Include resting periods between trials to prevent fatigue
- Counterbalance task conditions to control for order effects
4. Data Collection and Metrics
- Record continuous neural data throughout the session
- Log system outputs and timing with millisecond precision
- Collect subjective user feedback through standardized questionnaires
- Monitor for artifacts and system malfunctions
Table 2: Key Performance Metrics for Closed-Loop BCI Evaluation
| Metric Category | Specific Metrics | Target Values | Measurement Method |
|---|---|---|---|
| Information Transfer | Classification Accuracy, Bit Rate [31] | >70% accuracy, >30 bits/min | Online performance during closed-loop operation |
| System Responsiveness | Signal Delay, Feedback Latency [31] | <300 ms total latency | Timing synchronization across components |
| User Experience | Usability Score, Workload Index [31] | Subjectively reported | Standardized questionnaires (e.g., SUS, NASA-TLX) |
| Long-Term Stability | Performance Consistency, Signal Quality [31] | <15% performance degradation | Repeated measures across sessions |
5. Data Analysis
- Preprocess data with standardized pipelines
- Extract relevant features from neural signals
- Compute performance metrics for each experimental condition
- Conduct statistical tests to evaluate significance of findings
- Correlate neural measures with behavioral outcomes
This protocol emphasizes the gold standard of online evaluation, where system performance is assessed during real-time, closed-loop operation rather than through offline analysis alone [31].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Essential Research Materials for Closed-Loop BCI Development
| Item Category | Specific Examples | Function/Purpose |
|---|---|---|
| Signal Acquisition | EEG caps with Ag/AgCl electrodes, ECoG grid electrodes, Intracortical microelectrode arrays [30] [4] | Capture neural signals with appropriate spatial and temporal resolution for the research objectives |
| Signal Processing | MATLAB with EEGLAB, BCILAB, Python with MNE, Scikit-learn, TensorFlow [31] | Preprocess, feature extract, and classify neural signals in real-time with established algorithmic approaches |
| Stimulation Devices | Transcranial electrical stimulators (tDCS, tACS), Transcranial magnetic stimulators (TMS), Optogenetic laser systems [30] | Provide targeted neural modulation for closed-loop feedback interventions |
| Output Interfaces | Robotic arms (e.g., Baxter, KUKA), Functional electrical stimulators, Text speller interfaces [30] [8] | Translate decoded neural commands into functional actions in the physical or virtual environment |
| Experimental Control | Presentation software, Psychtoolbox, Unity3D, LabVIEW [31] | Precisely control task paradigms, timing, and data synchronization across system components |
| Biocompatible Materials | Polyimide-based substrates, Platinum-iridium electrodes, Conductive hydrogels [4] | Ensure long-term stability and safety for invasive or semi-invasive neural interfaces |
Advanced Implementation: A Two-Dimensional Framework for Signal Acquisition
Recent advances in BCI technology have necessitated more sophisticated frameworks for understanding signal acquisition. A two-dimensional perspective that simultaneously considers surgical invasiveness and sensor operating location provides valuable guidance for both clinical implementation and engineering design [4].
The surgical dimension classifies procedures based on their level of invasiveness: non-invasive (no anatomical trauma), minimal-invasive (trauma that spares brain tissue), and invasive (trauma affecting brain tissue at micron scale or larger) [4]. Parallel to this, the detection dimension categorizes sensors based on their operating location: non-implantation (on body surface), intervention (within natural body cavities), and implantation (within human tissue) [4].
This framework reveals critical trade-offs in BCI design. As systems move toward more invasive surgical procedures and deeper sensor implantation, signal quality theoretically improves due to proximity to neural sources and reduced interference from biological layers [4]. However, this comes with increased clinical risk, ethical considerations, and implementation challenges [4].
Figure 2: Two-Dimensional BCI Signal Acquisition Framework. This classification system simultaneously considers surgical procedures and sensor operating locations to guide BCI design and implementation decisions.
The closed-loop BCI system represents a sophisticated integration of four functionally distinct yet interdependent components: acquisition, processing, output, and feedback. The critical advantage of this architecture lies in its capacity for bidirectional adaptation, where both the user's brain and the system algorithms co-adapt to optimize performance over time. This dynamic interaction enables the system to function not merely as a passive communication channel, but as an active participant in a learning cycle that can induce neuroplasticity and enhance functional outcomes.
Future developments in closed-loop BCI technology will likely focus on improving the seamlessness of integration across these four components, enhancing the temporal precision of feedback delivery, and developing more sophisticated adaptive algorithms that can anticipate user intentions. Additionally, the growing emphasis on user-centered design principles necessitates comprehensive evaluation methods that assess not only technical performance metrics but also usability, user satisfaction, and quality of life impacts [31]. As these systems continue to evolve, they hold tremendous potential to transform approaches to neurological rehabilitation, cognitive augmentation, and human-computer interaction.
Signal Acquisition Technologies: From Scalp to Cortex
Brain-Computer Interface (BCI) technology facilitates direct communication between the brain and external devices, creating a non-muscular channel for interaction [6] [4]. The efficacy of any BCI system is fundamentally contingent upon its signal acquisition module, which bears the critical responsibility for detecting and recording cerebral signals [4]. A typical BCI system comprises four integral components: (1) signal acquisition, responsible for detecting and recording brain activity; (2) processing, which analyzes and decodes the recorded signals using specialized algorithms; (3) output, which executes the decoded intent through external devices; and (4) feedback, which closes the loop by informing the user of the system's interpretation and execution results [4].
The development of BCI systems is inherently interdisciplinary, necessitating collaboration between clinicians focused on surgical safety and engineers focused on signal performance [4]. This article elucidates a comprehensive two-dimensional framework for classifying BCI signal acquisition technologies, synthesizing surgical and engineering perspectives to provide researchers and developers with a structured understanding of current capabilities and trade-offs.
The Two-Dimensional Classification Framework
The proposed framework evaluates BCI signal acquisition techniques along two primary dimensions: the Surgery Dimension, which addresses the invasiveness of the procedure from a clinical perspective, and the Detection Dimension, which concerns the operational location of sensors from an engineering perspective [4]. This dual-axis approach enables a more nuanced analysis than unidimensional classifications.
Surgery Dimension: Invasiveness of Procedures
The surgery dimension categorizes techniques based on the anatomical trauma incurred during signal acquisition [4]. This classification directly impacts ethical considerations, implementation feasibility, and required clinical oversight.
Table 1: Classification Levels in the Surgery Dimension
| Classification Level | Anatomical Trauma Description | Clinical Requirements | Ethical & Implementation Considerations |
|---|---|---|---|
| Non-Invasive | No anatomically discernible trauma to the subject [4]. | Typically obviates continuous clinical oversight [4]. | Lower risk profile, suitable for wider populations; generally lower signal fidelity [4] [32]. |
| Minimally Invasive | Causes anatomically discernible trauma that spares brain tissue [4]. | Involvement of neurology/neurosurgery experts often required [4]. | Balanced trade-off; access to better signals than non-invasive without full craniotomy [4]. |
| Invasive | Causes anatomically discernible trauma at the micron scale or larger to brain tissue [4]. | Direct involvement of experienced neurosurgeons mandatory [4]. | Highest signal quality [33]; weighed against surgical risks and permanent implantation concerns [33] [4]. |
Detection Dimension: Operating Location of Sensors
The detection dimension classifies technologies based on the sensor's location during operation, which determines the theoretical upper limit of signal quality and influences biocompatibility risks [4].
Table 2: Classification Levels in the Detection Dimension
| Classification Level | Sensor Location & Operational Principle | Theoretical Signal Quality & Characteristics |
|---|---|---|
| Non-Implantation | Sensor operates on the surface of the body without entering natural cavities [4]. | Lower theoretical upper limit; analogous to "listening to a chorus from outside the building" where only large-scale neuronal sums are detectable amid noise [4]. |
| Intervention | Sensor leverages naturally existing cavities (e.g., blood vessels) without harming tissue integrity [4]. | Intermediate signal quality; sensors are closer to neural sources than non-implantation, but not embedded within tissue. |
| Implantation | Sensor is implanted within human tissue [4]. | Highest signal quality; minimal distance to signal source and fewest signal-degrading barriers [4]. Prone to tissue integration over time, complicating removal [4]. |
Figure 1: Two-Dimensional BCI Classification Framework. This diagram illustrates the core structure of the classification system, combining the Surgery and Detection dimensions.
Technical Analysis of BCI Modalities
Signal Characteristics Across the Framework
The fundamental differences between invasive and non-invasive signals are rooted in biophysics. Non-invasive techniques like EEG capture a superposition of predominantly low-frequency (<90 Hz) postsynaptic extracellular currents from millions of synchronously active pyramidal neurons, heavily filtered by the skull and scalp [33]. In contrast, invasive recordings provide access to a broader spectrum of neurophysiological processes, including action potentials (spiking activity up to several kHz) and local field potentials (LFPs), reflecting input, local processing, and output of cortical areas [33].
Table 3: Quantitative Comparison of Primary BCI Signal Acquisition Modalities
| Modality | Surgery Dimension | Detection Dimension | Spatial Resolution | Temporal Resolution | Key Signal Types | Primary Limitations |
|---|---|---|---|---|---|---|
| Electroencephalography (EEG) [33] [32] | Non-Invasive | Non-Implantation | ~1 cm | ~1-100 ms | Low-frequency (<90 Hz) postsynaptic currents | Susceptible to noise and artifacts; low spatial resolution [33]. |
| Digital Holographic Imaging (DHI) [34] | Non-Invasive | Non-Implantation | High (potential) | N/A (nanometer-scale tissue deformation) | Neural tissue deformation | Emerging technology; requires mitigation of physiological clutter [34]. |
| Vascular Stent Electrodes [4] | Minimally Invasive | Intervention | Higher than EEG | N/A | Local field potentials | Novel approach; long-term stability and biocompatibility under investigation. |
| Intracortical Electrodes [33] [4] | Invasive | Implantation | ~50-500 μm | <1 ms (for APs) | Action Potentials (APs), Local Field Potentials (LFPs) | Surgical risk; long-term signal stability and tissue response [33]. |
A critical advantage of intracortical approaches is the inherently higher possible information transfer rate, which is a key determinant for complex control tasks like operating robotic arms [33]. Furthermore, invasive BMIs offer unique opportunities for "sensation" by establishing a direct input channel to the brain via intracortical microstimulation, potentially restoring somatosensory feedback [33].
Detailed Experimental Protocols and Methodologies
Protocol: Non-Invasive AI-BMI for Robotic Arm Control
This protocol, derived from recent UCLA research, demonstrates how AI copilots can overcome the poor signal-to-noise ratio of non-invasive BCIs [35] [36].
- Participant Setup: Fit participant with a multi-channel EEG head cap according to the standard 10-20 system for electrode placement [36] [32].
- Calibration and Decoder Training:
- Instruct the participant to observe or imagine specific movements (e.g., hand grasping, cursor moving).
- Record raw EEG data synchronized with the observed or intended actions.
- Utilize a Convolutional Neural Network (CNN) to preprocess and extract features from the multichannel EEG time-series data [35].
- Train a ReFIT-like Kalman filter (KF) to map the extracted neural features to kinematic parameters (velocity, position) of the cursor or robotic arm [35].
- AI Copilot Integration:
- Closed-Loop Task Execution:
- In the "pick-and-place" task, the participant uses the AI-BMI to control a robotic arm. The BCI deciphers movement intentions, while the AI infers the overall goal (e.g., moving a specific block to a target location) and assists in task completion by refining the trajectory or providing stabilizing control [36].
- Performance Metrics: Quantify performance by task completion time and success rate, comparing results with and without AI assistance [36].
Figure 2: AI-BCI Experimental Workflow. This diagram outlines the protocol for non-invasive BCI systems enhanced with an AI copilot.
Protocol: Identifying Neural Signals via Digital Holographic Imaging
This protocol from Johns Hopkins APL addresses the challenge of identifying a novel non-invasive neural signal [34].
- System Configuration: Develop a digital holographic imaging (DHI) system with a laser source and a specialized camera sensitive enough to detect nanometer-scale deformations. The system must actively illuminate the neural tissue and record the scattered light to form a complex image [34].
- Signal Identification: Use the DHI system to spatially resolve changes in brain tissue velocity, identifying tissue deformation of tens of nanometers in height that correlates temporally with neural firing [34].
- Clutter Mitigation: Conduct fundamental tests over extended periods to distinguish the neural deformation signal from competing physiological noise (e.g., blood flow, heart rate, respiratory rate). This involves sophisticated signal processing akin to remote sensing techniques [34].
- Signal Validation: Correlate the detected tissue deformation signals with known markers of neural activity to validate that the signal is indeed a direct consequence of electrophysiological activity [34].
The Scientist's Toolkit: Research Reagent Solutions
Table 4: Essential Materials and Reagents for BCI Signal Acquisition Research
| Item | Function in Research | Example Application / Note |
|---|---|---|
| Multi-channel EEG System with Head Cap | Records electrical brain activity from the scalp surface [32]. | Foundation for non-invasive BCI research; often used with standard 10-20 placement system [32]. |
| Intracortical Multi-Electrode Arrays (e.g., Utah Array) | Records action potentials and local field potentials directly from neural tissue [33]. | Provides high-resolution data for invasive BCI paradigms; typically requires specialized surgical implantation [33]. |
| Digital Holographic Imaging (DHI) System | Detects nanometer-scale tissue deformations associated with neural activity [34]. | Emerging tool for non-invasive, high-resolution neural recording; used to identify novel signal sources [34]. |
| Convolutional Neural Network (CNN) Decoder | Processes and decodes raw, multichannel neural data (e.g., EEG) into control features [35]. | Key software tool for modern BCI decoding, improving performance by learning spatiotemporal patterns. |
| ReFIT-like Kalman Filter | Translates decoded neural features into smooth, continuous control signals for external devices [35]. | Algorithm critical for real-time, closed-loop BCI control, enhancing precision and usability. |
| Custom AI Copilot Platform | Infers user intent and assists in task completion by integrating BCI output with environmental context [35] [36]. | Compensates for low signal-to-noise ratio in non-invasive BCIs; often uses a camera-based vision system. |
The two-dimensional framework of surgical invasiveness and sensor location provides a comprehensive structure for classifying BCI signal acquisition technologies, illuminating the inherent trade-offs between signal fidelity, clinical risk, and practical implementation. The future of BCI lies in harmonizing these disciplinary perspectives, advancing technologies like minimally invasive interventions and sophisticated AI integration to break the performance-risk tradeoff [4] [35] [36]. By achieving equilibrium between these pivotal considerations, researchers can propel BCI technology forward, bolstering its effectiveness, safety, and dependability for a wider range of clinical and assistive applications.
Non-invasive brain signal acquisition technologies, particularly Electroencephalography (EEG) and functional Near-Infrared Spectroscopy (fNIRS), have become cornerstone methodologies in neuroscience research and brain-computer interface (BCI) development. These technologies enable researchers to capture neural activity without surgical intervention, making them essential tools for both clinical applications and basic scientific research. EEG measures the brain's electrical activity through electrodes placed on the scalp, providing direct insight into neural firing patterns with millisecond temporal resolution [37] [38]. In contrast, fNIRS employs near-infrared light to monitor hemodynamic responses associated with neural activity, offering better spatial resolution and greater tolerance to movement artifacts [37] [38]. The complementary nature of these modalities has led to growing interest in hybrid systems that leverage their respective strengths for improved brain activity decoding [39] [37].
The significance of these technologies extends across multiple domains, including neuroprosthetics, neurorehabilitation, cognitive neuroscience, and drug development [37] [40] [41]. For individuals with motor disabilities, EEG and fNIRS provide a vital communication pathway by detecting motor imagery (MI) signals that can control external devices [37] [42]. In pharmaceutical research, these technologies offer objective biomarkers for assessing treatment efficacy and understanding drug effects on brain function [40]. As the field advances, personalized BCIs are emerging that tailor signal acquisition and processing to individual user characteristics, enhancing system performance and user experience [41]. This technical guide examines the fundamental principles, methodological considerations, and practical applications of EEG and fNIRS technologies, with a specific focus on their integration within modern BCI systems.
Fundamental Principles and Physiological Basis
EEG: Electrical Neural Activity Acquisition
Electroencephalography (EEG) operates on the principle of detecting electrical potentials generated by synchronized neuronal activity in the brain. When pyramidal cells in the cerebral cortex fire in synchrony, they create post-synaptic potentials that propagate through various tissues (meninges, skull, scalp) before being detected by electrodes on the scalp surface [38]. These electrical signals represent the cumulative activity of millions of neurons, with primary contributions from cortical layers oriented perpendicular to the scalp surface [38]. The resulting voltage fluctuations, typically ranging from 10 to 100 microvolts, are captured over time, providing a direct measurement of neural electrical activity with exceptional temporal resolution in the millisecond range [38].
The international 10-20 system represents the standard methodology for electrode placement, ensuring consistent positioning across subjects and research studies [37]. This system defines electrode locations based on proportional distances between anatomical landmarks (nasion, inion, and preauricular points), facilitating reproducible measurements from specific brain regions [37]. To optimize signal conductivity, the electrode-scalp interface typically requires careful preparation, including scalp cleansing and application of conductive gels or pastes, though modern systems are increasingly utilizing dry electrodes for improved usability [37]. Despite these advancements, EEG signals remain susceptible to various artifacts including electrical line noise, muscle activity, eye blinks, and movement, necessitating sophisticated signal processing techniques to extract meaningful neural information [37].
fNIRS: Hemodynamic Response Monitoring
Functional Near-Infrared Spectroscopy (fNIRS) leverages the relative transparency of biological tissues to near-infrared light (typically 650-950nm) to measure hemodynamic changes associated with neural activity. This technology operates on the principle of neurovascular coupling, where neural activation triggers increased metabolic demand, leading to localized changes in cerebral blood flow and oxygenation [37] [38]. fNIRS systems emit near-infrared light through the scalp and skull into cortical tissue, where it undergoes scattering and absorption before being detected by optodes placed on the scalp surface [38].
The fundamental measurement in fNIRS involves quantifying changes in oxygenated hemoglobin (HbO) and deoxygenated hemoglobin (HbR) concentrations, as these chromophores exhibit distinct absorption spectra in the near-infrared range [37]. When neural activity increases in a specific brain region, cerebral blood flow to that region typically rises disproportionately to oxygen consumption, resulting in increased HbO and decreased HbR concentrations [38]. This hemodynamic response unfolds over several seconds, following a characteristic time course that begins approximately 2 seconds after neural activation and peaks at 5-8 seconds [38]. Unlike EEG, which measures neuronal activity directly, fNIRS captures metabolic consequences of neural activity, providing an indirect measure of brain function with superior spatial resolution but slower temporal response [38].
Table 1: Fundamental Principles of EEG and fNIRS
| Feature | EEG | fNIRS |
|---|---|---|
| Measured Signal | Electrical potentials from synchronized neuronal firing | Changes in hemoglobin oxygenation (HbO and HbR) |
| Physiological Basis | Post-synaptic potentials of pyramidal neurons | Neurovascular coupling (hemodynamic response) |
| Temporal Resolution | Milliseconds (direct neural transmission) | Seconds (slow hemodynamic response) |
| Spatial Resolution | Limited (cm-range) due to volume conduction | Moderate (cortical surface, ~1-2.5cm depth) |
| Depth Sensitivity | Cortical surface | Outer cortex (1-3cm) |
| Signal Source | Electrical activity of neurons | Metabolic activity (blood oxygenation) |
Neurovascular Coupling and Integrated Brain Activity
The relationship between EEG and fNIRS signals is mediated by neurovascular coupling, the fundamental process that links neural activity to subsequent hemodynamic changes. This complex biological mechanism involves interactions between neurons, astrocytes, and vascular cells, ultimately resulting in increased blood flow to active brain regions [38]. While EEG captures the initial electrical activity of neurons, fNIRS detects the metabolic consequences of this activity, creating complementary information streams about brain function [39]. Understanding this relationship is crucial for interpreting data from both modalities and for designing effective hybrid BCI systems that leverage their temporal and spatial strengths [39] [37].
The hemodynamic response function measured by fNIRS represents the integrated activity of populations of neurons over time, making it particularly suitable for investigating sustained cognitive processes, emotional states, and workload [38]. In contrast, EEG's millisecond-scale temporal resolution makes it ideal for capturing rapid neural dynamics associated with stimulus processing, motor planning, and transient cognitive events [37] [38]. The combination of these modalities provides a more comprehensive picture of brain activity than either approach alone, enabling researchers to connect fast electrical events with their slower metabolic consequences across specific brain regions [39] [37].
Diagram 1: Signaling Pathways of EEG and fNIRS
Technical Methodologies and Experimental Protocols
EEG Signal Acquisition Protocols
Establishing robust EEG acquisition protocols requires careful attention to multiple technical factors to ensure signal quality and reproducibility. The standard electrode placement follows the international 10-20 system or high-density variants (10-10, 10-5 systems), which provide systematic coverage of scalp regions corresponding to underlying cerebral cortex [37]. For motor imagery paradigms, electrodes are typically concentrated over sensorimotor areas (C3, Cz, C4 according to the 10-20 system), as these regions generate event-related desynchronization/synchronization (ERD/ERS) patterns during imagined movements [37] [42]. Proper skin preparation and electrode application are critical for achieving impedances below 5-10 kΩ, which minimizes noise and improves signal quality [37].
Modern EEG systems sample signals at rates typically between 250-2000 Hz, with anti-aliasing filters to prevent signal distortion [42]. The raw EEG signal contains various neural oscillations of interest (delta: 0.5-4 Hz, theta: 4-8 Hz, alpha: 8-13 Hz, beta: 13-30 Hz, gamma: >30 Hz) that can be extracted through digital filtering techniques [42]. For motor imagery classification, the mu rhythm (8-12 Hz) and beta rhythm (13-30 Hz) over sensorimotor cortex are particularly relevant, as they exhibit characteristic suppression during movement imagination [37] [42]. Experimental protocols typically involve timed cues prompting subjects to imagine specific movements (e.g., hand grasping, foot movement) while maintaining minimal physical movement to avoid contamination with electromyographic artifacts [42].
fNIRS Signal Acquisition Protocols
fNIRS experimental design requires careful consideration of source-detector geometry, optode placement, and task timing to effectively capture hemodynamic responses. Optodes are typically arranged in specific arrays over regions of interest, with source-detector distances of 2-4 cm to ensure sufficient penetration depth (1.5-3 cm) into the cortical surface [38]. Shorter separation channels (0.5-1 cm) may be incorporated to measure superficial scalp hemodynamics for signal correction [38]. For motor imagery studies, optodes are commonly placed over prefrontal and motor cortices to capture activation patterns associated with cognitive planning and movement imagination [37].
fNIRS systems employ continuous-wave, frequency-domain, or time-domain techniques, with continuous-wave systems being most common due to their relative simplicity and cost-effectiveness [38]. These systems typically use two or more wavelengths (e.g., 690nm and 830nm) to distinguish between HbO and HbR based on their differential absorption characteristics [38]. The modified Beer-Lambert law is applied to convert light intensity measurements into concentration changes of hemoglobin species [38]. Experimental tasks must account for the slow hemodynamic response, incorporating sufficient trial duration (typically 10-30 seconds) and appropriate inter-trial intervals to allow the signal to return to baseline [37] [38].
Hybrid EEG-fNIRS Acquisition Framework
Integrating EEG and fNIRS requires addressing technical challenges related to hardware synchronization, sensor placement, and signal co-registration. Simultaneous acquisition necessitates specialized caps that accommodate both electrodes and optodes without interference, often using predefined fNIRS-compatible openings in high-density EEG caps [38]. Hardware synchronization is typically achieved through external triggers (TTL pulses) or shared clock systems that timestamp data from both modalities [38]. The international 10-20 system provides a common framework for positioning both types of sensors, facilitating anatomical correspondence between electrical and hemodynamic measurements [38].
Table 2: Experimental Protocol Parameters for Motor Imagery Paradigms
| Parameter | EEG Protocol | fNIRS Protocol | Hybrid Protocol |
|---|---|---|---|
| Task Duration | 4-7 seconds | 10-30 seconds | 10-20 seconds |
| Inter-Trial Interval | 2-4 seconds | 15-30 seconds | 15-25 seconds |
| Key Regions of Interest | C3, Cz, C4 (Sensorimotor) | Prefrontal, Motor Cortex | Prefrontal, Sensorimotor Cortex |
| Signal Features of Interest | ERD/ERS in Mu/Beta rhythms | HbO increase, HbR decrease | Combined ERD/ERS and HbO/HbR |
| Sample Rate | 250-2000 Hz | 1-50 Hz | EEG: 250-2000 Hz, fNIRS: 1-50 Hz |
| Typical Trial Count | 40-100 per condition | 20-40 per condition | 30-60 per condition |
Diagram 2: Hybrid EEG-fNIRS Experimental Workflow
Advanced Signal Processing and Classification
EEG Signal Processing Pipelines
EEG signal processing involves multiple stages to transform raw signals into informative features for classification. preprocessing typically begins with filtering (e.g., 0.5-40 Hz bandpass for motor imagery) to remove irrelevant frequency components and line noise [42]. Artifact removal techniques address contamination from eye movements, muscle activity, and cardiac signals, with independent component analysis (ICA) being particularly effective for isolating and removing these non-neural sources [42]. For motor imagery paradigms, spatial filtering methods like Common Spatial Patterns (CSP) enhance discriminability between different mental states by maximizing variance for one class while minimizing it for another [42].
Feature extraction focuses on capturing relevant neural patterns, with time-frequency decompositions (wavelet transforms) commonly used to quantify event-related desynchronization and synchronization (ERD/ERS) in specific frequency bands [42]. More recently, deep learning approaches employing convolutional neural networks (CNNs) and long short-term memory (LSTM) networks have demonstrated excellent performance by automatically learning discriminative features from raw or minimally processed EEG signals [39] [42]. These methods can capture complex spatial-temporal patterns that may be difficult to characterize with handcrafted features, though they typically require larger datasets for training [42].
fNIRS Signal Processing Pipelines
fNIRS processing addresses unique challenges including physiological noise, motion artifacts, and the slow hemodynamic response. preprocessing typically involves converting raw light intensity measurements to optical density, then to concentration changes of HbO and HbR using the modified Beer-Lambert law [38]. Bandpass filtering (0.01-0.3 Hz) removes physiological noise from cardiac (∼1 Hz), respiratory (0.2-0.3 Hz), and very low-frequency drift components [38]. Motion artifacts present significant challenges and are typically addressed using wavelet-based methods, spline interpolation, or principal component analysis [38].
Feature extraction for fNIRS often focuses on statistical properties of the hemoglobin concentration changes, including mean, maximum, minimum, slope, and variance values within specific time windows [39]. More advanced approaches employ temporal filtering and utilize the entire hemodynamic response curve as input to machine learning models [39]. For classification, support vector machines (SVMs) and linear discriminant analysis (LDA) have been widely used, though recent studies have successfully applied deep learning architectures including convolutional neural networks and gated recurrent units (GRUs) to capture temporal dynamics of the hemodynamic response [39].
Multimodal Fusion Strategies
Integrating EEG and fNIRS data presents significant opportunities but also challenges due to their different physiological origins, temporal characteristics, and spatial properties. Fusion approaches generally operate at three levels: data-level fusion (combining raw signals), feature-level fusion (concatenating extracted features), and decision-level fusion (combining classifier outputs) [39]. Feature-level fusion typically involves normalizing and concatenating features from both modalities before feeding them to a classifier, though this must address the significant differences in temporal resolution between modalities [39] [38].
Decision-level fusion has shown particular promise, with methods like Dempster-Shafer Theory (DST) providing a mathematical framework for combining evidence from both modalities while accounting for uncertainty [39]. Recent advances include modeling uncertainty using Dirichlet distribution parameter estimation followed by evidence reasoning processes to fuse outputs from modality-specific classifiers [39]. These approaches have demonstrated significant performance improvements, with one study reporting 83.26% accuracy for motor imagery classification, representing a 3.78% improvement over state-of-the-art unimodal methods [39]. Deep learning architectures have also been developed for end-to-end multimodal fusion, with dual-pathway networks extracting spatiotemporal features from EEG using temporal convolution and attention mechanisms while processing fNIRS with spatial convolution and GRU networks [39].
The Scientist's Toolkit: Research Reagents and Materials
Table 3: Essential Research Materials for EEG-fNIRS Experiments
| Item | Function/Application | Technical Specifications |
|---|---|---|
| EEG Electrodes | Electrical signal acquisition from scalp | Ag/AgCl composition; Disposable or reusable; Wet/gel/dry types; Impedance <5-10 kΩ |
| Conductive Gel/Paste | Facilitate electrical conductivity between scalp and electrodes | NaCl-based electrolyte; Ten20 paste, NeuroPrep gel; Viscosity optimized for application |
| fNIRS Optodes | Light emission and detection | Source LEDs/lasers (690nm, 830nm); Detector photodiodes; Source-detector distance: 2-4cm |
| International 10-20 Cap | Standardized sensor placement | Elastic cap with measured positions; Compatible with EEG electrodes and fNIRS optodes |
| Abrasive Skin Prep Gel | Reduce skin impedance for EEG | Mild abrasive with cleansing properties; Reduces impedance to acceptable levels |
| Head Measurement Calipers | Precise localization of 10-20 positions | Measuring nasion-to-inion, preauricular distances; Essential for reproducible placement |
| Optical Phantoms | fNIRS system validation and testing | Materials with known optical properties; Mimics light scattering in biological tissue |
| Signal Amplifiers | Boost weak physiological signals | High input impedance, low noise; 24-bit resolution for EEG; Gain: 10,000-50,000 |
| Data Synchronization Unit | Temporal alignment of multimodal data | TTL pulse generation; Shared clock system; Simultaneous trigger to all devices |
Applications in Brain-Computer Interfaces and Neuroresearch
Motor Imagery and Neuroprosthetic Control
Motor imagery-based BCIs represent a primary application area for both EEG and fNIRS, particularly for individuals with motor impairments or limb loss. These systems decode the user's intention to move a limb without actual physical movement, enabling control of prosthetic devices, computer interfaces, or rehabilitation robots [37] [42]. EEG-based MI-BCIs typically exploit event-related desynchronization in mu (8-12 Hz) and beta (13-30 Hz) rhythms over sensorimotor areas during movement imagination [37] [42]. fNIRS-based approaches detect hemodynamic changes in prefrontal and motor cortices associated with the cognitive planning of movements [37]. While offline studies often achieve higher classification accuracy due to fewer time constraints and richer data processing, recent advancements in machine learning have improved the feasibility of online MI decoding for real-time applications [37].
Hybrid EEG-fNIRS systems have demonstrated superior performance for MI classification compared to unimodal approaches by combining EEG's temporal precision with fNIRS's spatial specificity [39] [37]. For lower-limb motor imagery specifically, which presents unique challenges due to smaller cortical representation areas, these multimodal approaches have shown particular benefit [42]. Recent studies report that 85% of lower-limb MI investigations applied machine or deep learning classifiers such as SVM, CNN, and LSTM, while 65% incorporated multimodal fusion strategies to improve classification accuracy, signal interpretability, and real-time application potential [42]. These developments are paving the way for clinically viable BCIs that are accessible, adaptable, and suitable for real-world neurorehabilitation contexts [42].
Personalized BCIs and Clinical Translation
The emergence of personalized brain-computer interfaces (pBCIs) represents a significant advancement in non-invasive neural signal acquisition and application. pBCIs address individual differences in physiological and mental states, sensations, perceptions, imageries, cognitive thinking activities, and brain structures and functions that complicate the application of general BCIs [41]. Personalization approaches include customized paradigm design, tailored signal processing algorithms (including personalized channel selection and feature extraction), and adaptive classification models that accommodate individual characteristics [41]. These approaches have demonstrated improved performance and user experience compared to one-size-fits-all BCI frameworks [41].
Clinical applications of EEG and fNIRS extend beyond motor restoration to include cognitive assessment, emotional state monitoring, and neurofeedback interventions [40] [41]. In mental health treatment, these technologies provide objective biomarkers for diagnosing conditions and monitoring treatment response, particularly in antipsychotic drug development where they can quantify neural effects of pharmacological interventions [40]. The movement toward portable, low-power BCI devices optimized for fewer channels is making these technologies increasingly viable for real-world clinical applications and home-based rehabilitation programs [42]. However, methodological variability and lack of standardization persist across studies, posing barriers to widespread clinical implementation [42].
Comparative Analysis and Future Directions
Table 4: Comprehensive Comparison of EEG and fNIRS Technologies
| Characteristic | EEG | fNIRS | Hybrid Approach |
|---|---|---|---|
| Temporal Resolution | Milliseconds (excellent for fast neural dynamics) | Seconds (limited by hemodynamic response) | Combines millisecond (EEG) with second-scale (fNIRS) |
| Spatial Resolution | Limited (cm-range) due to volume conduction | Moderate (better than EEG) for cortical areas | Enhanced through fNIRS spatial specificity |
| Portability | High (lightweight wireless systems available) | High (wearable formats common) | Moderate (increasingly portable systems) |
| Movement Tolerance | Low (susceptible to motion artifacts) | High (relatively robust to movement) | Moderate (depends on EEG component) |
| Setup Complexity | Moderate (requires conductive gel/scalp prep) | Moderate (optode placement with minimal contact) | High (multiple sensor types, synchronization) |
| Depth Sensitivity | Cortical surface | Outer cortex (1-2.5 cm depth) | Comprehensive cortical coverage |
| Cost Considerations | Generally lower | Generally higher, especially high-density | Highest (requires both systems) |
| Ideal Applications | Fast cognitive tasks, ERPs, sleep research | Naturalistic studies, child development, sustained tasks | Comprehensive brain monitoring, advanced BCIs |
The future development of EEG and fNIRS technologies is trending toward increased integration, miniaturization, and intelligence. Multimodal fusion of EEG and fNIRS continues to be an active research area, with advances in deep learning and evidence theory improving classification performance for various BCI applications [39]. Portable, low-power systems optimized for fewer channels are becoming increasingly sophisticated, enhancing the practical applicability of these technologies outside controlled laboratory environments [42]. The development of standardized, open-access datasets and protocols represents a critical need for accelerating translation and adoption into broader clinical and research contexts [42].
Personalized BCIs represent another significant frontier, with systems increasingly adapting to individual user characteristics, capabilities, and needs [41]. This includes user-centered paradigm design, customized signal processing pipelines, and adaptive interfaces that optimize performance for specific individuals [41]. As these technologies continue to evolve, they hold promise for transforming neurorehabilitation, restoring communication and mobility for individuals with severe disabilities, and providing new insights into brain function and organization. Continued coordination between researchers, clinicians, and engineers remains essential for addressing persistent challenges related to signal quality, system reliability, and user acceptability in real-world settings.
Brain-Computer Interface (BCI) technology has evolved significantly, with a prominent trend towards minimizing the invasiveness of neural signal acquisition systems. Traditionally, a trade-off existed where high-fidelity signals required invasive intracortical or subdural electrodes implanted via open-brain surgery, which carried risks of tissue injury, inflammation, and long-term stability issues [43]. Minimally invasive and endovascular approaches represent a paradigm shift, aiming to preserve high-quality signal acquisition while substantially reducing surgical morbidity and improving chronic biocompatibility. This whitepaper provides an in-depth technical analysis of two key approaches within this domain: the Stentrode, an endovascular BCI, and minimally invasive Electrocorticography (ECoG) techniques. Framed within the broader context of BCI basic working principles and signal acquisition research, this review details the architectural design, operational mechanisms, and experimental protocols that underpin these transformative technologies.
Core Working Principles and Signal Acquisition in BCI
The efficacy of any BCI system is fundamentally contingent upon its signal acquisition module, which is responsible for detecting and recording cerebral signals [4]. A typical BCI architecture comprises four integral components: Signal Acquisition, Processing (which includes feature extraction and classification), Output (to control external devices), and Feedback, which closes the loop [2] [4]. The quality of the acquired signal—its resolution, signal-to-noise ratio (SNR), and bandwidth—directly dictates the performance and capabilities of the entire system.
To systematically evaluate and compare BCI signal acquisition technologies, a two-dimensional framework that considers both the surgical procedure (invasiveness) and the sensor operating location is essential [4].
- Surgery Dimension (Invasiveness of Procedures): This perspective, crucial for clinicians, classifies procedures based on the anatomical trauma incurred:
- Non-invasive: No anatomically discernible trauma (e.g., scalp EEG).
- Minimally invasive: Causes anatomically discernible trauma but does not impact brain tissue (e.g., endovascular Stentrode).
- Invasive: Causes anatomically discernible trauma at the micron scale or larger to brain tissue (e.g., intracortical microelectrodes, some ECoG arrays) [4].
- Detection Dimension (Operating Location of Sensors): This perspective, critical for engineers, classifies technologies based on the sensor's final location:
- Non-implantation: Sensors operate on the surface of the body.
- Intervention: Sensors leverage naturally existing cavities (e.g., blood vessels) without harming original tissue.
- Implantation: Sensors are located within human tissue [4].
This framework clarifies the positioning of the Stentrode as a minimally-invasive, interventional technology, while many ECoG arrays are classified as invasive, implantation devices, though newer designs are reducing the associated surgical footprint.
The Stentrode: An Endovascular Brain-Computer Interface
Architectural Design and Operating Principles
The Stentrode system, developed by Synchron, is a fully implantable endovascular neural interface that enables chronic electrocorticographic (ECoG) recording without penetrating cortical tissue [43] [44]. Its design leverages the vascular system as a natural conduit to position electrodes near the motor cortex.
The system's architecture consists of three primary components:
- Nitinol Stent Scaffold: A self-expanding stent fabricated from a nickel-titanium alloy (nitinol), chosen for its superelasticity and shape-memory properties. The scaffold, with optimized dimensions of approximately 40 mm in length and 8 mm in diameter, serves as both the delivery vehicle and mechanical backbone, providing stable anchorage within the superior sagittal sinus (SSS) [43].
- Thin-Film Electrode Array: Sixteen platinum-iridium electrodes, coated with iridium oxide to enhance charge injection capacity and reduce polarization, are lithographically patterned onto a flexible polyimide film. This array is adhesively bonded to the stent's luminal surface, ensuring the electrodes maintain close apposition to the venous endothelium [43].
- Implantable Receiver-Transmitter Unit (IRTU): A hermetically sealed, titanium-encased subcutaneous unit located in a subclavicular pocket. The IRTU is connected via a flexible, insulated lead tunneled from the internal jugular vein. It performs low-noise amplification and digitization of neural signals and handles wireless data transmission and power reception via inductive coupling [43].
Table 1: Technical Specifications of the Stentrode System
| Component | Material/Technology | Key Properties & Function |
|---|---|---|
| Stent Scaffold | Nitinol (Ni-Ti alloy) | Superelasticity, shape memory, provides radial force for stability within vessel [43]. |
| Electrodes | Platinum-Iridium coated with Iridium Oxide | High corrosion resistance, inert electrochemistry, enhanced charge injection capacity [43]. |
| Electrode Substrate | Polyimide film | Flexibility, biocompatibility, insulation for metal traces [43]. |
| Insulation Layer | Parylene-C | Biocompatible dielectric coating to prevent electrical crosstalk and signal leakage [43]. |
| Telemetry & Power | Bluetooth Low Energy (BLE), Inductive Coupling | Wireless data transmission and power delivery, eliminating need for percutaneous wires [43]. |
Biocompatibility and the Endovascular Interface
A critical aspect of the Stentrode's design is its chronic biostability within the dynamic venous environment. Post-implantation, a natural endothelialization process occurs, where migrating endothelial cells envelop the stent struts and electrode surfaces within approximately four weeks [43]. Preclinical ovine models, which have cortical hemodynamics comparable to humans, demonstrated that this process stabilizes the electrode-vessel interface without inducing thrombus formation or intimal hyperplasia, while preserving venous patency for up to 190 days [43]. To mitigate early thromboembolic risk, a dual antiplatelet regimen (e.g., aspirin and clopidogrel) is typically administered for the first 90 days [43].
Experimental Protocol and Preclinical-to-Clinical Translation
The development and validation of the Stentrode followed a structured translational pathway.
- Preclinical Ovine Model: The endovascular approach was first established in sheep due to their neurovascular anatomical similarities to humans [44]. The implantation protocol involves:
- Access: Percutaneous access is gained via the internal jugular vein.
- Navigation: A microcatheter is navigated under fluoroscopic guidance into the superior sagittal sinus, positioned over the primary motor cortex.
- Deployment: The Stentrode is delivered through the catheter and self-expands into apposition with the vessel wall.
- Lead Routing & Unit Implantation: The connected lead is routed back through the jugular vein and tunneled subcutaneously to the IRTU in the chest wall [43].
- Signal Acquisition & Analysis: Neural signals are recorded, with a focus on the high-gamma band, which is known to correlate with motor activity. The signals are amplified, digitized (at ≥1 kHz per channel), and transmitted wirelessly to an external unit for decoding [43].
- Clinical Translation: Building on positive preclinical safety and feasibility data, clinical trials were initiated. As reported, six patients with amyotrophic lateral sclerosis (ALS) successfully used the Stentrode system for digital communication, demonstrating stable long-term signal acquisition and control of external devices [44].
Minimally Invasive Electrocorticography (ECoG) Approaches
Advancements in High-Density and Flexible ECoG Arrays
While traditional ECoG involves placing electrode arrays directly on the brain surface via a craniotomy, recent innovations focus on minimizing the surgical footprint and improving the interface's quality. High-Density Microelectrode Arrays (HD-MEAs) represent a significant leap, enabled by advances in microfabrication and complementary metal-oxide-semiconductor (CMOS) technology [45]. These arrays can feature thousands of electrodes per mm², allowing for unprecedented spatial resolution in mapping neural activity across broad cortical areas, from local field potentials (LFPs) to action potential propagation [45].
A parallel development is the creation of Flexible High-Density Microelectrode Arrays (FHD-MEAs). These devices use soft, compliant substrates (e.g., polyimide, parylene) to conform to the brain's gyral surface, reducing the mechanical mismatch between rigid electronics and neural tissue. This conformity improves signal stability, reduces inflammatory response, and enables chronic recording capabilities [46].
A Novel Minimally Invasive Implantation Technique
A groundbreaking approach to ECoG implantation was recently demonstrated, enabling the placement of a thin, flexible microelectrode array through a small slit in the skull, avoiding a large craniotomy [47]. This method involves:
- Small Craniotomy: A narrow linear slit or burr hole is created in the skull.
- Array Insertion: The thin, flexible electrode array is rolled or folded and then slid through the opening onto the surface of the dura mater or the brain itself.
- Cortical Coverage: Once inserted, the array unfurls to cover a broad cortical area, providing high-resolution neural recording and stimulation capabilities [47].
This technique was validated in both animal studies and human pilot studies, showing the ability to record and stimulate neural activity across broad cortical areas with a significantly reduced surgical impact [47].
Table 2: Comparison of BCI Signal Acquisition Approaches
| Parameter | Endovascular Stentrode | Minimally Invasive ECoG (via slit) | Traditional ECoG (via craniotomy) |
|---|---|---|---|
| Surgical Classification | Minimally Invasive [4] | Invasive [4] | Invasive [4] |
| Surgical Procedure | Endovascular (Venous) | Small Slit Craniotomy | Large Craniotomy |
| Spatial Resolution | Moderate (Sacrificed for access) [43] | High to Very High [47] | High |
| Typical Signal Type | ECoG (High-Gamma) [43] | ECoG, High-Resolution Local Field Potentials [47] | ECoG |
| Chronic Biostability | Endothelialization preserves venous patency [43] | Conformal, flexible design reduces inflammation [46] | Risk of encapsulation & signal degradation |
| Key Advantage | Avoids open-brain surgery; lower procedural risk [43] [44] | High-resolution data with minimal surgical footprint [47] | Established clinical procedure |
| Reported Clinical Use | ALS patients for digital communication [44] | Pilot human studies [47] | Extensive (e.g., epilepsy monitoring) |
Experimental Methodologies and Data Analysis
Signal Processing Workflow
The journey from raw neural signal to a device command is a multi-stage computational process. After acquisition, signals undergo preprocessing (e.g., filtering to remove noise and artifacts like line noise) [2]. Subsequent steps include:
- Feature Extraction: Critical electrophysiological features are pulled from the signal. For motor-based BCIs, this often involves analyzing the power in specific frequency bands, such as sensorimotor rhythms or high-gamma activity (70-200 Hz) [43] [2]. Time-domain features like event-related potentials can also be used.
- Feature Classification: Machine learning algorithms (e.g., Support Vector Machines, deep learning models) are trained to recognize patterns in the extracted features that correspond to the user's intent (e.g., imagining hand movement vs. rest) [2] [4].
- Feature Translation: The classified intent is translated into actionable commands for an external device, such as a computer cursor or a robotic arm [2].
A key performance metric for BCI systems is the Information Transfer Rate (ITR), measured in bits per minute. ITR is a function of both the speed and the accuracy of the system [48].
The Scientist's Toolkit: Key Research Reagents and Materials
Table 3: Essential Materials for Minimally Invasive BCI Research
| Item / Reagent | Function in Research & Development |
|---|---|
| Nitinol Tubing | Base material for laser-cutting self-expanding stent scaffolds; provides essential superelasticity [43]. |
| Platinum-Iridium Sputtering Targets | Source for physical vapor deposition (sputtering) to create thin-film, corrosion-resistant electrodes [43]. |
| Polyimide Precursors | Forms the flexible, biocompatible substrate and insulation layer for the electrode array [43] [46]. |
| Parylene-C Deposition System | Applies a uniform, pinhole-free, biocompatible dielectric coating for neural implants [43]. |
| Dual Antiplatelet Therapy (Aspirin, Clopidogrel) | Pharmacological regimen to mitigate early thromboembolic risk in endovascular implants [43]. |
| CMOS-MEA Chips | Provides the high-density electrode arrays and integrated electronics for high-resolution ECoG recording and stimulation [45]. |
The landscape of brain-computer interfacing is being reshaped by the concerted drive toward minimizing invasiveness without compromising signal fidelity. The Stentrode exemplifies this through its innovative endovascular approach, leveraging the vascular system to create a stable biointerface and demonstrating tangible clinical benefits for patients with severe paralysis. Concurrently, advances in minimally invasive ECoG, particularly through high-density, flexible arrays deployed via small cranial openings, are pushing the boundaries of spatial resolution and cortical coverage while reducing surgical morbidity. Both approaches, situated within the functional framework of BCI signal acquisition, offer distinct yet complementary pathways forward. Future progress will hinge on the continued interdisciplinary collaboration between clinicians and engineers to further optimize signal processing, enhance long-term biocompatibility, and refine implantation procedures, ultimately accelerating the translation of these powerful technologies from the laboratory to broader clinical and consumer applications.
Invasive intracortical interfaces represent a cornerstone of modern brain-computer interface (BCI) technology, enabling direct communication between the brain and external devices with unparalleled resolution. These systems translate neural activity into commands to restore functional independence for patients with paralysis and other neurological disorders [49]. The evolution toward high-channel-count implants has been driven by the need to capture neural representations with greater fidelity, moving from broad population recordings to single-neuron resolution across multiple brain regions [50]. This progression addresses the fundamental working principle of BCIs: that the richness of decodable information increases with both the spatial coverage and density of recording sites [51]. Contemporary invasive interfaces now routinely incorporate thousands of microelectrodes, pushing the boundaries of neuroscientific discovery and clinical application while introducing new challenges in design, manufacturing, and surgical implementation [50].
Types of Invasive Microelectrode Arrays
Invasive microelectrode arrays (MEAs) can be categorized into three primary classes based on their structural composition and manufacturing methodologies. Each type offers distinct advantages and limitations for specific research and clinical applications.
Table 1: Comparison of Major Invasive Microelectrode Array Types
| Array Type | Key Materials | Manufacturing Process | Channel Count Range | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Microwire-based | Tungsten, Platinum-Iridium, Stainless Steel, Carbon Fiber [50] | Manual assembly, bundling, twisting [50] | Single wires to 100s+ [50] | Simple, low-cost, highly customizable [50] | Limited density, geometric variability [50] |
| Silicon-based (Michigan Probes) | Silicon, Platinum, Iridium Oxide [50] | Microfabrication (photolithography, etching) [50] | 10s to 100s [50] | High spatial precision, integrated electronics [50] | Stiffness causing tissue damage, limited chronic stability [50] |
| Flexible Substrate-based | Polyimide, Parylene, Graphene [50] | Thin-film deposition, photolithography [50] | 100s to 1000s [51] [50] | Conformable, reduced tissue damage, high channel count [51] [50] | Buckling during insertion, requires stiffeners [50] |
Microwire-Based Microelectrode Arrays
Microwire-based MEAs represent the earliest and most straightforward approach to intracortical recording. These arrays consist of fine metallic wires, typically less than 100 μm in diameter, insulated along their shafts with exposed conductive tips for neural signal acquisition [50]. Configurations range from single-wire implants to complex two-dimensional (2D) and three-dimensional (3D) arrays, including specialized tetrodes and stereotrodes where multiple wires are twisted together to improve single-unit isolation [50]. The primary advantages of microwire arrays include their relative simplicity, low cost, and high customizability for targeting specific brain regions. However, they suffer from limitations in achieving high-density configurations and exhibit significant geometric variability due to their manual assembly processes [50].
Silicon-Based Microelectrode Arrays
Silicon-based MEAs, epitomized by the "Michigan probe," leverage microelectromechanical systems (MEMS) fabrication techniques to create precisely patterned neural interfaces with integrated electronics [50]. These arrays benefit from photolithographic processes that enable complex electrode geometries with multiple recording sites along each shank, significantly improving spatial sampling density compared to microwire arrays. The rigid nature of silicon facilitates precise insertion into neural tissue without buckling. However, this same rigidity creates a mechanical mismatch with brain tissue, leading to chronic inflammatory responses and signal degradation over time [50]. Additionally, the fabrication complexity and material costs are substantially higher than those for microwire arrays.
Flexible Substrate-Based Microelectrode Arrays
Recent advances have focused on developing flexible MEAs using polymers such as polyimide and parylene as substrate materials [50]. These arrays offer superior biocompatibility and reduced tissue damage due to their mechanical properties, which more closely match those of brain tissue [51]. The flexible nature enables conformal contact with cortical surfaces and minimizes chronic immune responses, leading to improved long-term recording stability [51]. High-density 1,024-channel thin-film microelectrode arrays have been demonstrated with electrode pitches as small as 400 μm, enabling large-scale cortical mapping [51]. The primary challenge for flexible MEAs is their tendency to buckle during insertion, necessitating the use of biodegradable or temporary stiffeners and specialized insertion techniques [50].
Design Considerations and Key Parameters
The development of high-performance intracortical interfaces requires careful optimization of multiple interdependent design parameters that directly influence recording quality, biocompatibility, and long-term stability.
Table 2: Key Design Parameters for Intracortical Microelectrode Arrays
| Parameter | Typical Range/Options | Impact on Performance | Considerations |
|---|---|---|---|
| Electrode Material | Platinum, Iridium, Gold, Carbon Nanotubes, Graphene [50] | Impedance, charge injection, biocompatibility [50] | Biocompatibility, stability, manufacturing feasibility [50] |
| Electrode Size | 20-200 μm diameter [51] | Signal-to-noise ratio, spatial resolution [51] | Smaller electrodes have higher impedance but better spatial resolution [51] |
| Inter-Electrode Spacing | 300-400 μm [51] | Cross-talk, spatial sampling density [51] | Tighter spacing reduces unique information but increases correlation [51] |
| Array Configuration | 2D grid, 3D array, conformable sheet [50] | Brain coverage, surgical accessibility [50] | Target brain region, implantation method [50] |
| Interconnect Technology | Wire bonding, flexible printed circuits [50] | Reliability, channel count scalability [50] | Surgical integration, long-term reliability [50] |
Material Selection and Biocompatibility
Material choice critically influences the electrical, mechanical, and biological performance of intracortical interfaces. Traditional materials include noble metals such as platinum, iridium, and gold, which offer excellent conductivity and biostability [50]. Recent research has explored carbon-based materials including carbon fibers, carbon nanotubes, and graphene, which provide low impedance, high charge injection capacity, and improved biocompatibility [50]. Equally important is the selection of insulation materials, with parylene-C and polyimide being the most common choices for flexible arrays due to their excellent dielectric properties, flexibility, and biocompatibility [50]. Surface modifications and coatings are often employed to further enhance biocompatibility and reduce foreign body responses.
Electrode Density and Scalability
The drive toward higher channel counts represents a central theme in modern MEA development, with contemporary systems now exceeding 1,000 channels [51]. High-density configurations enable comprehensive sampling of neural populations while maintaining single-neuron resolution. The optimal balance between electrode size and spacing depends on the specific application requirements—smaller electrodes (20-50 μm) provide higher spatial resolution but exhibit higher impedance, which can degrade signal-to-noise ratio [51]. Recent studies have demonstrated that inter-electrode pitches of 300-400 μm provide a favorable compromise, minimizing cross-talk while maximizing unique information capture across adjacent channels [51]. Scalability challenges primarily involve interconnect density, packaging, and the management of the resulting data bandwidth, which can exceed gigabytes per minute in ultra-high-density systems.
Manufacturing Processes and Fabrication
The fabrication methodologies for invasive MEAs vary significantly across different array types, each requiring specialized processes and equipment.
Microwire Array Fabrication
Microwire array manufacturing begins with selection and preparation of appropriate wire materials, typically metals such as tungsten, stainless steel, or platinum-iridium alloys [50]. The wires are precision-cut to length, followed by insulation removal at the tips using mechanical stripping, laser ablation, or electrochemical etching. For multi-wire arrays, individual wires are meticulously assembled into specific configurations using custom jigs and fixtures, then bonded together with epoxy or other adhesives [50]. Recent advances include the development of carbon fiber electrodes with diameters as small as 5-10 μm, requiring specialized handling and assembly techniques due to their extreme flexibility [50].
Silicon Array Fabrication
Silicon-based MEAs are manufactured using standardized MEMS processes, beginning with silicon wafers that undergo multiple stages of photolithographic patterning, thin-film deposition, and etching [50]. The process typically involves depositing and patterning conductive traces (often gold or platinum) followed by insulation layers (silicon nitride or silicon dioxide) that are selectively opened at recording sites. Deep reactive ion etching (DRIE) is then used to define the probe shank geometry and release the devices from the wafer [50]. Advanced silicon processes enable the integration of on-chip circuitry for signal amplification and multiplexing, significantly reducing the number of required external connections [50].
Flexible Array Fabrication
Flexible MEAs are fabricated using thin-film processes similar to those employed in the flexible electronics industry. The process typically begins with deposition of a sacrificial layer onto a rigid carrier wafer, followed by sequential deposition and patterning of the polymer substrate, metal traces, and insulation layers [50]. Photolithography and etching techniques define the electrode sites and interconnect patterns. Laser ablation or etching subsequently releases the finished arrays from the carrier wafer [50]. Electrode sites are often modified with electrodeposited platinum black or other nanomaterials to reduce impedance and improve signal acquisition [50].
Surgical Implantation Techniques
The successful deployment of intracortical interfaces requires specialized surgical techniques and instrumentation that minimize tissue damage while ensuring precise electrode placement.
Craniotomy-Based Approaches
Traditional implantation methods involve performing a craniotomy—the surgical removal of a bone flap from the skull to expose the dura mater covering the brain [51]. After incising the dura, MEAs are inserted directly into the cortical tissue using mechanical microdrives or pneumatic insertion systems [50]. For silicon-based and rigid microwire arrays, insertion speeds typically range from 1-100 μm/s, with slower speeds generally resulting in reduced acute tissue damage [50]. Durable cranial chambers are often installed to protect the implant and provide long-term access to the brain for chronic recordings [50].
Minimally Invasive Delivery
Recent advances have focused on developing less traumatic implantation techniques. The "cranial micro-slit" approach uses precision sagittal saw blades to create 500-900 μm wide incisions in the skull, enabling subdural insertion of thin-film arrays without full craniotomy [51]. This technique, demonstrated in porcine models and human cadavers, can be completed in under 20 minutes per array and facilitates placement over large cortical areas with minimal tissue disruption [51]. For flexible arrays, temporary stiffeners made from biodegradable materials such as silk or sucrose are often employed to prevent buckling during insertion [50]. Additional strategies include mechanical support from solid microneedles, shuttle devices, and hydraulic or pneumatic insertion systems that provide the necessary force for penetration while minimizing tissue dimpling [50].
Intraoperative Guidance and Validation
Precise electrode placement is verified using multimodal imaging and neurophysiological monitoring. Intraoperative fluoroscopy or computed tomography provides real-time visualization of array deployment [51]. Micro-endoscopy allows direct observation of the cortical surface during insertion [51]. Functional validation involves recording spontaneous and evoked neural activity to confirm placement in target regions, with somatosensory, visual, and motor cortex locations verified through appropriate stimulation paradigms [51].
Signal Processing and Neural Decoding
The utility of high-channel-count intracortical interfaces depends on sophisticated signal processing pipelines that extract meaningful information from raw neural data.
Signal Acquisition and Preprocessing
Neural signals acquired through intracortical electrodes span multiple frequency bands, each carrying distinct physiological information. Action potentials (300-5,000 Hz) provide single-neuron resolution, while local field potentials (1-300 Hz) reflect population-level activity [51]. Acquisition systems must handle substantial data rates, with 1,024-channel arrays sampling at 30 kHz requiring continuous data streaming exceeding 60 MB/s [51]. Preprocessing stages typically include common-average referencing to reduce common-mode noise, bandpass filtering to isolate frequency bands of interest, and artifact rejection algorithms to remove electrical interference from environmental sources or stimulation artifacts in closed-loop systems [51].
Feature Extraction and Dimensionality Reduction
Modern decoding approaches leverage population-level neural activity patterns rather than single-unit responses alone. Dimensionality reduction techniques such as principal component analysis (PCA), factor analysis (FA), and Gaussian-process factor analysis (GPFA) extract latent variables that capture prominent co-fluctuation patterns across neural populations [52]. These methods enable visualization of neural trajectories through state spaces that evolve dynamically during behavior [52]. For clinical applications, features are typically extracted in sliding windows of 100-500 ms, balancing temporal resolution with decoding accuracy [11].
Decoding Algorithms and Performance
Machine learning algorithms translate neural features into control commands for external devices. Support vector machines (SVMs) effectively classify discrete states, while convolutional neural networks (CNNs) and recurrent neural networks (RNNs) have shown superior performance for continuous kinematic decoding [11]. Transfer learning approaches address the challenge of neural signal non-stationarity by adapting decoder parameters across sessions without requiring complete recalibration [11]. Recent studies with high-density arrays have demonstrated accurate decoding of somatosensory, visual, and volitional walking activity, with decoding accuracy improving as a function of both spatial coverage and electrode density [51].
Experimental Protocols and Methodologies
Rigorous experimental protocols are essential for characterizing the performance and safety of intracortical interfaces in both acute and chronic settings.
In Vitro Characterization
Before implantation, MEAs undergo comprehensive electrical and mechanical testing. Electrochemical impedance spectroscopy measures interface impedance across relevant frequencies (10 Hz-10 kHz), with typical values ranging from 802±30 kΩ for 20 μm electrodes to 8.25±0.65 kΩ for 380 μm electrodes [51]. Cyclic voltammetry assesses charge storage capacity and charge injection limits, critical parameters for stimulation-capable electrodes [50]. Accelerated aging tests evaluate material stability through repeated sterilization cycles and extended immersion in phosphate-buffered saline at body temperature [50]. Mechanical testing includes bend cycling for flexible arrays and insertion force measurement using precision load cells [50].
In Vivo Validation
Animal studies, typically conducted in porcine or non-human primate models, evaluate recording performance, stimulation efficacy, and biological responses [51]. Neural recording quality is quantified using signal-to-noise ratio, single-unit yield, and recording stability metrics [51]. For motor decoding applications, animals perform controlled behavioral tasks while neural activity is recorded, enabling quantification of decoding accuracy for kinematic parameters [51]. Sensory evoked potentials are recorded following controlled stimulation to assess the functional integrity of neural circuits [51]. Focal neuromodulation capabilities are validated through cortical stimulation at sub-millimeter scales, with behavioral responses or electrophysiological changes serving as outcome measures [51].
Biocompatibility and Safety Assessment
Formal biocompatibility studies compare test implants against control devices in randomized cohorts with subacute (7-day) and chronic (42-day) endpoints [51]. Histopathological analysis includes hematoxylin and eosin staining to assess general tissue architecture and immunohistochemical markers such as Iba1 for microglial activation [51]. Neuronal density and glial scarring around implant sites are quantified to evaluate tissue damage and the foreign body response [51]. Blood-brain barrier integrity is assessed using immunoglobulin G extravasation assays [51]. For clinical applications, these preclinical studies follow Good Laboratory Practice standards and inform regulatory submissions [51].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Essential Research Reagents and Materials for Intracortical Interface Research
| Category | Specific Items | Function/Application | Examples/Specifications |
|---|---|---|---|
| Electrode Materials | Platinum-Iridium wire [50] | High-fidelity neural recording | Excellent biocompatibility, stable chronic performance [50] |
| Carbon fiber electrodes [50] | Single-unit recording | 5-10 μm diameter, high flexibility, minimal tissue damage [50] | |
| Polyimide substrates [50] | Flexible array foundation | Biocompatible polymer for thin-film arrays [50] | |
| Fabrication Supplies | Parylene-C coating [50] | Dielectric insulation | Conformal coating for neural implants [50] |
| Photoresists [50] | Lithographic patterning | AZ5214, SU-8 for MEMS fabrication [50] | |
| Silicon wafers [50] | Michigan probe substrate | High-purity, specific crystal orientation [50] | |
| Surgical Equipment | Cranial micro-slit blades [51] | Minimally invasive access | 500-900 μm wide sagittal saw blades [51] |
| Biodegradable stiffeners [50] | Flexible array insertion | Silk, sucrose, PEG-based temporary supports [50] | |
| Pneumatic insertion systems [50] | Controlled implantation | Precision insertion with minimal tissue dimpling [50] | |
| Signal Processing Tools | DataHigh GUI [52] | Neural trajectory visualization | MATLAB-based tool for high-dimensional data [52] |
| Elephant toolkit [53] | Electrophysiology analysis | Python library for spike train analysis [53] | |
| Brainstorm [53] | Multimodal data integration | Open-source application for MEG/EEG/ECoG analysis [53] |
Future Directions and Challenges
The field of invasive intracortical interfaces continues to evolve toward higher channel counts, improved biocompatibility, and expanded clinical applications. Next-generation systems aim to incorporate tens of thousands of electrodes while minimizing tissue damage through further miniaturization and flexible materials [50]. Bidirectional interfaces that combine high-resolution recording with precise microstimulation will enable closed-loop neuroprosthetic systems for restoration of sensory and motor functions [11]. Significant challenges remain in developing fully implantable wireless systems with sufficient data bandwidth and power efficiency for chronic human use [49]. Clinical translation requires demonstration of long-term reliability and safety through rigorous preclinical testing and controlled clinical trials [51]. As these technologies advance, they will continue to revolutionize our understanding of neural coding while providing transformative solutions for individuals with neurological disorders.
A Brain-Computer Interface (BCI) is a system that measures brain activity and converts it in real-time into functionally useful outputs, changing the ongoing interactions between the brain and its external or internal environments [1]. These systems create a direct link between the brain and external devices, representing a substantial advancement in human-machine interaction [7] [54]. All BCI systems share a similar processing pipeline consisting of signal acquisition, processing and decoding, output generation, and a feedback loop [1].
The efficacy of BCI systems is largely contingent upon progress in signal acquisition methodologies, which can be classified into non-implantation, intervention, and implantation techniques [7] [6]. Invasive approaches record neural activity directly from the brain surface or cortex, while non-invasive methods measure activity from the scalp surface [1] [55]. The interdependence between interaction paradigms and signal acquisition technologies means that innovations in one domain often propel progress in the other [7].
Figure 1: Core BCI Processing Pipeline. This diagram illustrates the fundamental closed-loop architecture of brain-computer interfaces, from signal acquisition to device control with integrated feedback.
BCI for Motor Restoration
BCI technology offers significant potential for motor restoration in patients with neurological conditions such as spinal cord injury, stroke, and amyotrophic lateral sclerosis (ALS) [55]. By interpreting neural signals and converting them into control commands, BCIs can bypass damaged neural pathways, offering therapeutic potential for restoring movement and interaction with the environment [55].
Current applications include motor restoration via robotic exoskeletons and functional electrical stimulation, allowing paralyzed individuals to control digital and physical devices with their thoughts [1] [55]. Several companies are advancing this field with various technological approaches, as summarized in Table 1.
Table 1: Comparative Analysis of Leading BCI Systems for Motor Restoration and Communication
| Company/Institution | Technology Approach | Invasiveness Level | Key Application | Clinical Trial Status (2025) | Key Specifications |
|---|---|---|---|---|---|
| Neuralink | Ultra-high-bandwidth implantable chip with thousands of micro-electrodes | Invasive | Control of digital and physical devices by individuals with paralysis | 5 patients in trials [1] | Records from more neurons than prior devices; coin-sized implant |
| Synchron | Stentrode device delivered via blood vessels | Minimally invasive | Computer control for texting and communication | Four-patient trial completed; moving toward pivotal trial [1] | No skull drilling; placed in superior sagittal sinus via jugular vein |
| Blackrock Neurotech | Neuralace flexible lattice array | Invasive | Motor restoration and communication | Expanding trials including in-home tests [1] | Flexible design reduces scarring compared to traditional Utah arrays |
| Paradromics | Connexus BCI with 421 electrodes | Invasive | Speech restoration for paralyzed individuals | FDA approval for clinical study in November 2025 [56] | High-channel-count implant for ultra-fast data transmission |
| Precision Neuroscience | Layer 7 ultra-thin electrode array | Minimally invasive | Communication for ALS patients | FDA 510(k) clearance for up to 30 days implantation [1] | "Brain film" inserted through slit in dura; conforms to cortical surface |
Experimental Protocol: Motor Imagery BCI for Upper Limb Rehabilitation
Objective: To assess the efficacy of motor imagery-based BCI systems for upper limb motor function recovery in stroke patients.
Methodology:
- Participant Selection: Recruit patients with upper limb paresis following ischemic or hemorrhagic stroke (3-12 months post-onset)
- Signal Acquisition: Apply 64-channel EEG cap according to 10-20 international system with focused electrodes over sensorimotor cortex
- Task Paradigm: Implement Graz BCI protocol with alternating cues for hand opening/closing imagery vs. rest states
- Signal Processing:
- Bandpass filtering (8-30 Hz) for sensorimotor rhythm extraction
- Common Spatial Pattern (CSP) algorithm for feature extraction
- Linear Discriminant Analysis (LDA) for classification of motor imagery states
- Feedback System: Provide visual feedback via virtual hand movement on screen proportional to classification confidence
- Assessment Metrics: Fugl-Meyer Assessment (FMA) for upper extremity, Action Research Arm Test (ARAT), and EEG-based performance metrics (accuracy, Cohen's kappa)
Duration: 18 sessions, 3 times weekly for 6 weeks, 45 minutes per session
BCI for Communication Restoration
BCI technology represents an innovative frontier for restoring communication abilities in individuals with severe speech and motor impairments [54] [57]. Recent advances have demonstrated that BCIs can detect speech attempts and even inner speech from speech-impaired patients, offering hope for restoring rapid communication [57].
The brain's motor cortex contains regions that control the muscular movements that produce speech. BCIs using tiny arrays of microelectrodes surgically implanted in the brain's surface can record neural activity patterns directly from the brain [57]. These signals are then fed via a cable to a computer algorithm that translates them into intended speech or text output.
Experimental Protocol: Inner Speech Decoding for Communication
Objective: To develop a BCI system that decodes inner speech from neural signals to enable communication without physical speech attempts.
Methodology:
- Participant Selection: Individuals with severe speech and motor impairments (e.g., advanced ALS, locked-in syndrome) with implanted microelectrode arrays
- Signal Acquisition: Utilize microelectrode arrays (Blackrock Neurotech or similar) implanted in speech motor cortex
- Task Paradigm:
- Overt Attempted Speech: Patient attempts to speak words despite physical limitations
- Covert Inner Speech: Patient imagines speaking words without any physical movement
- Password-Protected Mode: Implementation of security phrase ("as above, so below") to prevent accidental decoding of private thoughts [57]
- Neural Feature Extraction:
- Time-domain spike sorting and thresholding
- Local field potential analysis in frequency bands (high-gamma, 70-150 Hz)
- Phoneme-level representation mapping through deep learning networks
- Decoding Algorithm:
- Machine learning training to recognize repeatable patterns of neural activity associated with phonemes
- Recurrent neural network (RNN) with long short-term memory (LSTM) layers for temporal sequence modeling
- Integration of language model priors for sentence completion and error correction
- Output Generation: Text display and synthesized speech output with adjustable speed and voice characteristics
Assessment Metrics: Word error rate, characters per minute, information transfer rate (bits per minute), user satisfaction scales
Figure 2: Inner Speech BCI Decoding Pathway. This workflow illustrates the parallel processing of inner and overt speech signals with integrated privacy protection.
BCI in Neurorehabilitation
BCIs represent an emerging advancement in neurological rehabilitation, enabling direct communication between the brain and external devices to aid recovery in individuals with neurological impairments [55]. BCIs can be classified into invasive, semi-invasive, non-invasive, or hybrid types, each with distinct applications in rehabilitation settings [55].
Current applications include cognitive enhancement through neurofeedback and attention training, communication tools for individuals with severe physical limitations, and motor recovery via robotic exoskeletons and functional electrical stimulation [55]. Advances in EEG signal acquisition, machine learning, wearable and wireless systems, and integration with virtual reality are enhancing the clinical utility of BCIs by improving accuracy, adaptability, and usability [55].
Experimental Protocol: Closed-Loop BCI for Stroke Rehabilitation
Objective: To evaluate the efficacy of a closed-loop BCI system with functional electrical stimulation (FES) for upper limb motor recovery in chronic stroke patients.
Methodology:
- Participant Selection: Chronic stroke patients (>6 months) with moderate to severe upper limb impairment
- System Configuration:
- 32-channel EEG system with dry electrodes for rapid setup
- EMG recording from forearm flexor and extensor muscles
- FES system with programmable stimulation parameters
- Real-time processing unit with closed-loop control algorithms
- Intervention Protocol:
- Motor Attempt Detection: EEG-based detection of movement intention from sensorimotor rhythms
- Triggered FES: Application of functional electrical stimulation to target muscles upon detection of movement intention
- Adaptive Stimulation: Adjustment of FES parameters based on EMG response and performance metrics
- Visual Feedback: Virtual reality display showing intended vs. actual movement
- Training Paradigm:
- Object manipulation tasks (reaching, grasping, releasing)
- Progressive difficulty adjustment based on performance
- Incorporation of gamified elements to enhance engagement
- Outcome Measures:
- Primary: Box and Blocks Test, Fugl-Meyer Assessment
- Secondary: Motor Activity Log, EEG-EMG coherence, resting-state functional connectivity
- Neuroplasticity Measures: Transcranial magnetic stimulation (TMS) for motor evoked potentials
Duration: 24 sessions over 8 weeks, 60 minutes per session, with 3-month follow-up assessment
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Research Materials and Technologies for BCI Development
| Research Tool | Function/Application | Example Products/Technologies |
|---|---|---|
| Microelectrode Arrays | Neural signal recording from cortical surface | Blackrock Neurotech Utah Array, Neuralink chip, Paradromics Connexus |
| Endovascular Electrodes | Minimally invasive signal acquisition through blood vessels | Synchron Stentrode |
| Flexible Neural Interfaces | Biocompatible electrodes reducing tissue scarring | Axoft Fleuron material, Precision Neuroscience Layer 7 |
| Graphene-Based Electrodes | High-resolution signal recording with superior material properties | InBrain Neuroelectronics graphene electrodes |
| EEG Acquisition Systems | Non-invasive brain signal recording | 64-channel wet/dry EEG systems with active electrodes |
| BCI Software Toolkits | Signal processing and machine learning algorithms | BCI Toolbox (Python), OpenVibe, BCILAB |
| Neurostimulation Systems | Closed-loop modulation of neural activity | Functional electrical stimulation (FES) systems |
| Virtual Reality Platforms | Immersive feedback environments for neurorehabilitation | Custom VR interfaces integrated with BCI systems |
Quantitative Analysis of BCI Performance
Quantitative assessment of BCI performance is essential for evaluating system efficacy and guiding improvements. Table 3 summarizes key performance metrics across different BCI applications based on current literature and clinical studies.
Table 3: Quantitative Performance Metrics for Core BCI Applications
| Application Domain | Performance Metrics | Current Performance Levels | Research Targets |
|---|---|---|---|
| Communication Restoration | Character per minute (CPM) | 50-90 CPM (attempted speech) [57] | >100 CPM with inner speech |
| Word Error Rate (WER) | <10% for limited vocabularies [57] | <5% for large vocabulary | |
| Accuracy (Phoneme/Word) | ~99% accuracy in lab settings [1] | >95% in real-world environments | |
| Motor Restoration | Movement Classification Accuracy | 70-90% for binary tasks [58] | >95% for multi-class tasks |
| Information Transfer Rate | 20-35 bits/min [55] | >50 bits/min | |
| Clinical Improvement (FMA-UE) | 5-8 points after BCI therapy [55] | >10 point improvement | |
| Neurorehabilitation | Response Latency | <0.25 seconds for detection [1] | <0.1 seconds |
| User Adaptation Rate | 20% potential increase with optimal task selection [58] | 30%+ with adaptive systems |
BCI technology has demonstrated significant potential across three core neurological applications: motor restoration, communication, and neurorehabilitation. The field stands roughly where gene therapies did in the 2010s or heart stents in the 1980s: on the cusp of graduating from experimental status to regulated clinical use, driven by a mix of startup innovation, academic research, and patient demand [1].
Future directions include the development of personalized, closed-loop, and home-based systems, enabled by interdisciplinary collaboration among clinicians, engineers, neuroscientists, and policymakers [55]. Improved hardware that enables more neurons to be recorded and is fully implantable and wireless will increase BCIs' accuracy, reliability and ease of use, with several companies working on hardware components expected to become available within the next few years [57]. With continued research and ethical implementation, BCIs have the potential to transform neurorehabilitation and greatly enhance patient outcomes and quality of life [55].
Overcoming Technical Hurdles: Signal Quality, Biocompatibility, and Data Processing
The field of Brain-Computer Interfaces (BCIs) is fundamentally governed by a critical engineering and clinical trade-off: the relationship between the fidelity of acquired neural signals and the degree of surgical invasiveness and risk required to obtain them [59]. Higher-resolution signals that provide detailed insights into neural processing typically require physical proximity to neural tissue, necessitating more invasive implantation procedures that carry greater surgical risk [2]. Conversely, non-invasive approaches eliminate surgical risk but yield signals with substantially lower spatial resolution and signal-to-noise ratios, limiting their decoding capabilities and applications [60]. This paper examines the technical foundations of this trade-off within the context of BCI basic working principles and signal acquisition research, providing researchers with a framework for selecting appropriate methodologies based on target applications.
BCI Fundamentals and Signal Acquisition Modalities
Core BCI Architecture and Working Principles
At its essence, a BCI is a system that measures central nervous system (CNS) activity and converts it into artificial output that replaces, restores, enhances, supplements, or improves natural CNS output [59]. The classical BCI pipeline comprises three fundamental components: (1) signal acquisition hardware that measures brain activity, (2) signal processing software that decodes this data, and (3) an output device that executes commands or provides feedback [2] [60]. This creates a closed-loop system where brain activity is continuously measured, interpreted, and translated into functional outputs [11].
BCIs can be functionally categorized into read-out systems that decode neural signals to infer user intent, and write-in systems that deliver electrical or optical stimulation to neural tissue to create artificial sensations or modulate neural activity [61]. The technical requirements and ethical considerations differ significantly between these functional types, particularly regarding their placement along the invasiveness-fidelity spectrum.
The Signal Acquisition Frontier: Technical Specifications
The quality of neural signals acquired by a BCI is fundamentally constrained by its signal acquisition method [62]. The following table summarizes the key technical characteristics of predominant signal acquisition modalities in current BCI research:
Table 1: Technical Specifications of BCI Signal Acquisition Modalities
| Modality | Spatial Resolution | Temporal Resolution | Invasiveness Level | Primary Signal Types | Key Advantages | Key Limitations |
|---|---|---|---|---|---|---|
| EEG [2] [60] | ~1 cm | ~1-100 ms | Non-invasive | Field potentials | Safe, portable, affordable | Low SNR, spatial resolution |
| ECoG [62] | ~1 mm | ~1-10 ms | Embedded (on dura) | Local field potentials, high-frequency broadband | Higher SNR than EEG, less drift | Requires craniotomy |
| Utah Array [1] [62] | ~50-100 μm | <1 ms | Intracortical (penetrating) | Single-unit activity, multi-unit activity | Direct neural firing data | Tissue damage, signal decline over time |
| Neuropixels [62] | ~10-20 μm | <1 ms | Intracortical (penetrating) | Single-unit activity across layers | Massive parallel recording (1000+ channels) | Complex implantation, data volume challenges |
| Endovascular (Stentrode) [1] [63] | ~1 mm | ~1-10 ms | Minimally invasive (via blood vessels) | Local field potentials | Avoids open brain surgery | Limited to vessel-adjacent regions |
| fNIRS [60] | ~1 cm | ~1-5 seconds | Non-invasive | Hemodynamic responses | Safe, portable | Indirect measure, slow temporal response |
The relationship between these modalities can be visualized through the following conceptual framework:
Diagram 1: BCI Invasiveness-Reliability Trade-off
Categorizing BCI Invasiveness and Surgical Risk Profiles
A Proposed Semantic Framework for BCI Risk Assessment
The terminology describing BCI invasiveness has historically been vague, with terms like "minimally invasive" applied to devices with substantially different risk profiles [59]. To address this ambiguity, a three-tiered categorization system has been proposed that better accommodates surgical footprint and attendant clinical risks [59] [64]:
- Non-invasive: BCI components do not penetrate the body (e.g., EEG headsets, fNIRS caps) [59]
- Embedded: Components are penetrative but not deeper than the inner table of the skull (e.g., ECoG grids placed on the dura) [59]
- Intracranial: Components are located within the inner table of the skull and possibly within the brain volume (e.g., Utah Arrays, Neuropixels probes) [59]
This framework provides a more precise language for discussing BCI technologies, particularly within the context of surgical procedures and risk-benefit analyses for specific clinical populations [59].
Comparative Risk Analysis Across BCI Categories
The surgical risk profile varies significantly across BCI categories, as detailed in the following table:
Table 2: Surgical Risk Profiles and Long-Term Considerations by BCI Category
| BCI Category | Representative Technologies | Surgical Procedure Requirements | Primary Acute Risks | Long-Term Stability & Biocompatibility |
|---|---|---|---|---|
| Non-invasive | EEG, fNIRS [2] [60] | None | None | Stable, but susceptible to environmental noise |
| Embedded | ECoG grids [62], Precision Neuroscience Layer 7 [1] | Craniotomy for placement on cortical surface | Surgical site infection, cerebrospinal fluid leak | Moderate; less tissue damage but subject to encapsulation |
| Intracranial (Penetrating) | Utah Array [62], Neuralink [1] | Craniotomy with penetration of parenchyma | Intracerebral hemorrhage, neural tissue damage | Poor; chronic foreign body response leads to glial scarring |
| Minimally Invasive | Synchron Stentrode [1] [63] | Endovascular delivery via jugular vein | Vessel injury, thrombosis | Unknown long-term viability in blood vessels |
Each category presents distinct risk-benefit considerations that must be balanced against the clinical need and performance requirements [59]. For patients with severe disabilities, higher risks may be justified by the potential for restored function, whereas different risk calculus applies to augmentation applications in healthy populations [65].
Experimental Methodologies and Technical Protocols
Standardized Experimental Workflow for BCI Signal Acquisition
Research in BCI signal acquisition follows a systematic methodology to ensure reproducible results. The following diagram outlines a generalized experimental workflow applicable across the fidelity-invasiveness spectrum:
Diagram 2: BCI Signal Acquisition Experimental Workflow
Key Research Reagent Solutions for BCI Research
The following table details essential research reagents and materials used in advanced BCI experimentation, particularly for intracortical approaches:
Table 3: Essential Research Reagents and Materials for BCI Signal Acquisition Studies
| Research Reagent/Material | Function/Application | Technical Specifications | Example Use Cases |
|---|---|---|---|
| Utah Array [62] | Intracortical microelectrode array for single-unit recording | 100 silicon microelectrodes in 10×10 grid; 1-1.5 mm length | Chronic recording in motor cortex for prosthetic control [1] |
| Neuropixels Probes [62] | High-density neural recording probes for large-scale sampling | ~1000 recording sites on single shank; CMOS-based design | Large-scale network dynamics studies across brain regions |
| Flexible Polymer Substrates [62] | Biocompatible electrode materials to reduce tissue response | Polyimide or parylene-C substrates; flexible geometry | Next-generation arrays minimizing micromotion-induced inflammation |
| Anti-inflammatory Coatings [62] | Surface treatments to improve biocompatibility and signal stability | Drug-eluting coatings (e.g., dexamethasone) | Chronic implants to extend functional electrode lifetime |
| ECoG Grids [2] [62] | Subdural electrode arrays for cortical surface recording | Various configurations (e.g., 8×8, 16×16); platinum-iridium contacts | Mapping epileptic networks; speech decoding studies |
| Wireless Data Transmission Systems [62] | Fully implantable systems for chronic recording | Integrated amplification & multiplexing; RF or infrared transmission | Preclinical testing of untethered BCI operation |
Emerging Approaches and Future Directions
Innovations in Minimizing the Invasiveness-Fidelity Trade-off
Several innovative approaches are emerging that aim to deliver high-fidelity signals while minimizing surgical risk. Endovascular BCIs like the Stentrode represent a promising middle ground, deploying electrode arrays via blood vessels to achieve proximity to neural tissue without open brain surgery [1] [63]. Early clinical results demonstrate the feasibility of this approach for achieving sufficient signal quality for device control while potentially mitigating surgical risks associated with craniotomy [1].
Concurrently, materials science advances are producing increasingly flexible and biocompatible electrode arrays that reduce the chronic foreign body response responsible for signal degradation over time [62]. These include ultra-thin conformable arrays that minimize mechanical mismatch with brain tissue, such as Precision Neuroscience's Layer 7 cortical interface [1]. The field is moving toward "bio-integrative" electrodes designed to form functional interfaces with neural tissue rather than merely being biocompatible [62].
Signal Processing Advances to Compensate for Acquisition Limitations
Parallel advancements in artificial intelligence and machine learning are helping to compensate for limitations in signal acquisition. Modern decoding algorithms, including convolutional neural networks (CNNs) and transfer learning (TL) approaches, can extract meaningful information from noisier signals, potentially reducing the fidelity requirements for functional BCIs [11]. These techniques are particularly valuable for non-invasive systems, where they can significantly improve classification accuracy despite lower signal-to-noise ratios [11].
The fundamental trade-off between signal fidelity and surgical invasiveness remains a core consideration in BCI research and development. While higher-fidelity signals generally require more invasive approaches with greater surgical risk, emerging technologies are gradually reshaping this relationship. Endovascular approaches, flexible bio-integrative electrodes, and advanced signal processing algorithms collectively represent promising pathways to mitigate this longstanding trade-off. For researchers and clinicians, selecting an appropriate BCI modality requires careful consideration of the target application, required information bandwidth, and acceptable risk profile. As the field advances, the ongoing refinement of both acquisition technologies and processing algorithms will continue to expand the feasible design space for brain-computer interfaces across the fidelity-invasiveness spectrum.
Brain-Computer Interface (BCI) technology establishes a direct communication pathway between the human brain and external devices, with its efficacy fundamentally contingent upon the quality of the acquired neural signals [23] [4]. At the heart of this challenge lies the signal-to-noise ratio (SNR), a critical metric that quantifies the relationship between the strength of target neural activity and the accompanying noise. Electroencephalography (EEG), a predominant non-invasive signal acquisition method, measures voltage fluctuations in the microvolt (µV) range, making it inherently susceptible to contamination from various biological and environmental artifacts [23] [66]. These artifacts, which include signals from ocular movements, muscle activity, cardiac rhythms, and environmental interference, can severely distort the neural information, complicating signal interpretation and potentially leading to misdiagnosis in clinical settings or faulty commands in assistive applications [67] [66]. Therefore, the development and implementation of robust strategies to combat noise and artifacts are not merely supplementary but are essential for advancing BCI systems toward reliable real-world and clinical use. This guide provides an in-depth examination of contemporary strategies for improving SNR, framed within the core working principles and signal acquisition research of BCIs.
A typical BCI system operates through a sequential pipeline comprising four main components: signal acquisition, processing (which includes preprocessing and classification), output, and feedback [23] [4]. The journey begins with signal acquisition, a module responsible for detecting and recording cerebral signals, whose performance fundamentally limits the entire system's capabilities [4].
The vulnerability of this process becomes clear when considering the nature of neural signals. Non-invasive signals, such as scalp EEG, are inherently weak and are recorded with a limited number of channels [23]. The resulting signals are a complex mixture of neural activity and noise. The table below categorizes the primary sources of noise and artifacts that degrade SNR in BCI systems.
Table 1: Classification of Common Noise and Artifacts in BCI Systems
| Category | Source Type | Specific Examples | Characteristics & Impact |
|---|---|---|---|
| Biological Artifacts | Physiological | Ocular movements (EOG), blinks, muscle activity (EMG), cardiac rhythms (ECG) | Overlap with neural signal frequencies (e.g., eye blinks <4 Hz, muscle activity >13 Hz) [66]. |
| Environmental Artifacts | External | Powerline interference (50/60 Hz), electrode pops, cable swings, magnetic induction | Introduce structured, high-amplitude noise that can obscure genuine neural activity [68]. |
| Motion Artifacts | Body Movement | Head tilts, walking, running, fasciculation | Cause significant, non-stationary signal corruption, especially challenging for mobile BCIs [68] [69]. |
The following diagram illustrates the core BCI workflow and the critical stage at which noise mitigation occurs.
Core Strategies for Noise and Artifact Mitigation
Advanced Signal Processing and Deep Learning Techniques
Traditional signal processing methods, such as Independent Component Analysis (ICA), regression-based approaches, and wavelet transforms, have long been foundational for artifact removal [68] [66]. These methods operate by decomposing the signal and estimating and subtracting the artifactual components. However, the field is rapidly evolving toward deep learning models, which can learn complex, non-linear relationships from data and provide end-to-end denoising solutions.
Several state-of-the-art deep learning architectures have demonstrated remarkable efficacy. The Artifact Removal Transformer (ART) is an end-to-end model that leverages transformer architecture to adeptly capture the transient, millisecond-scale dynamics characteristic of EEG signals. ART is trained on pseudo clean-noisy data pairs and can effectively remove multiple artifact sources simultaneously, significantly boosting subsequent BCI performance [70]. Another innovative approach, AnEEG, employs a Generative Adversarial Network (GAN) guided by Long Short-Term Memory (LSTM) layers. In this framework, a generator produces denoised signals, while a discriminator critiques them against clean data, leading to the generation of high-fidelity, artifact-free EEG signals [66]. Furthermore, GCTNet integrates a GAN with a parallel CNN and transformer network, enabling it to capture both global and temporal dependencies in the EEG data for superior denoising [66].
Table 2: Performance Comparison of Advanced Artifact Removal Models
| Model Name | Core Architecture | Key Innovation | Reported Performance Metrics |
|---|---|---|---|
| ART (Artifact Removal Transformer) [70] | Transformer | Holistic, end-to-end denoising for multiple artifact types. | Surpasses other deep-learning methods in MSE and SNR; improves BCI performance. |
| AnEEG [66] | GAN with LSTM | Adversarial training to generate pure EEG signals. | Lower NMSE/RMSE, higher CC, and improved SNR/SAR versus wavelet techniques. |
| GCTNet [66] | GAN + CNN + Transformer | Captures global and temporal dependencies simultaneously. | 11.15% reduction in RRMSE, 9.81 improvement in SNR over existing methods. |
| IMU-Enhanced LaBraM [69] | Fine-tuned Large Brain Model | Uses IMU data to inform spatial-channel attention for artifact removal. | More robust than ASR-ICA under diverse motion scenarios (walking, running). |
Multi-Modal Fusion and Sensor-Based Approaches
A powerful strategy for combating noise, particularly motion artifacts, involves multi-modal fusion. This approach leverages data from additional sensors to directly quantify and subsequently remove noise sources. Inertial Measurement Units (IMUs), which capture acceleration, angular velocity, and orientation, are exceptionally well-suited for this task [69].
Recent research fine-tunes large brain models, such as LaBraM, using a correlation attention mapping method. This model leverages spatial channel relationships in simultaneously recorded IMU data to identify motion-related artifacts in EEG signals. The IMU signals are projected into a shared latent space with EEG features, allowing an attention mechanism to pinpoint and gate motion-corrupted components effectively [69]. Earlier algorithms like iCanClean also utilized canonical correlation analysis (CCA) with IMU-derived references to enhance EEG quality across diverse conditions without extensive calibration [69]. The workflow for this multi-modal approach is illustrated below.
Paradigm Design and Signal Acquisition at the Source
While post-processing is crucial, a proactive strategy involves designing BCI paradigms and signal acquisition technologies to maximize SNR from the outset. A well-designed paradigm enhances the strength and separability of the user's brain intention response signals through specific tasks or patterns [23].
For example, Steady-State Visual Evoked Potentials (SSVEP) and code-modulated VEP (c-VEP) paradigms elicit strong, rhythmic brain responses in the visual cortex, which are inherently more distinguishable from background noise. Recent studies have successfully integrated c-VEP spellers with Mixed Reality (MR) headsets, achieving high information transfer rates (over 27 bits/min) and accuracy (over 96%) with minimal visual fatigue, showcasing how paradigm and hardware co-design can yield robust performance [71]. Furthermore, the fundamental choice of signal acquisition technology, spanning non-invasive, minimally invasive, and invasive methods, directly determines the theoretical upper limit of signal quality. As sensors move closer to the neural source (from scalp to intracranial), the signal strength increases, and vulnerability to biological and environmental artifacts decreases, albeit at the cost of higher surgical risk and ethical considerations [4].
Experimental Protocols and Methodologies
Protocol for Evaluating Deep Learning Denoising Models
To validate models like ART or AnEEG, a rigorous experimental protocol is required.
- Data Preparation and Preprocessing: Begin with a diverse dataset of raw EEG recordings contaminated with various artifacts (e.g., from public repositories like EEG DenoiseNet or PhysioNet). Preprocess the data by applying bandpass filtering (e.g., 0.1-75 Hz) and a notch filter (e.g., 50/60 Hz). Manually or semi-automatically (using ICA) create ground-truth "clean" EEG targets for supervised training [70] [66].
- Model Training: For a GAN-based model like AnEEG, train the generator to map noisy input EEG to clean EEG, while the discriminator is trained to distinguish the generator's output from the ground truth. Use a loss function that combines adversarial loss with a time-frequency domain loss (e.g., Mean Squared Error on signal and power spectral density) to ensure signal fidelity [66].
- Quantitative Evaluation: Evaluate the model on a held-out test set using multiple metrics:
- Normalized Mean Squared Error (NMSE) & Root Mean Squared Error (RMSE): Lower values indicate better agreement with the original, clean signal.
- Correlation Coefficient (CC): Higher values signify a stronger linear relationship with the ground truth.
- Signal-to-Noise Ratio (SNR) & Signal-to-Artifact Ratio (SAR): Higher values confirm successful denoising [66].
- BCI Performance Benchmark: The ultimate test is to apply the denoising model as a preprocessing step in a functional BCI paradigm (e.g., an SSVEP speller or MI task) and measure the improvement in classification accuracy and Information Transfer Rate (ITR) compared to using raw or classically processed signals [70].
Protocol for IMU-Enhanced Motion Artifact Removal
For evaluating multi-modal approaches, the protocol must incorporate motion data.
- Dataset Collection: Utilize a dataset such as the "Mobile BCI" dataset, which includes synchronized EEG and head-mounted IMU recordings during various activities (standing, walking, running) and BCI paradigms (SSVEP, ERP) [69].
- Data Synchronization and Alignment: Precisely synchronize the EEG and IMU data streams. Preprocess the EEG (filtering, resampling) and IMU signals (e.g., calibrating accelerometer and gyroscope data).
- Model Implementation: Fine-tune a pre-trained large brain model (e.g., LaBraM). Project the EEG and IMU data into a shared latent space using separate encoders. Train a correlation attention mechanism to map the relationships between IMU dynamics and EEG artifact patterns. An Artifact Gate Layer then uses this information to suppress motion-corrupted components [69].
- Comparative Analysis: Benchmark the performance of the IMU-enhanced model against established single-modality pipelines like Artifact Subspace Reconstruction followed by ICA (ASR-ICA). Evaluate performance across different motion intensities (slow walk vs. run) and time scales to demonstrate robustness [69].
The Scientist's Toolkit: Key Reagents and Materials
Table 3: Essential Research Tools for BCI Noise Mitigation Studies
| Tool / Material | Category | Primary Function in Research |
|---|---|---|
| High-Density EEG Systems (e.g., 32+ channels) | Hardware | Acquires neural data with high temporal resolution; essential for spatial filtering and source localization techniques. |
| Auxiliary IMU Sensors (9-axis: accelerometer, gyroscope, magnetometer) | Hardware | Provides reference signals for motion artifact removal via multi-modal fusion algorithms [69]. |
| ICA and ASR Algorithms | Software | Foundational signal processing tools for decomposing signals and identifying/removing artifact components; used as a baseline for comparison [68] [69]. |
| Transformer-based Models (e.g., ART) | Software/Algorithm | Provides state-of-the-art end-to-end denoising by capturing long-range temporal dependencies in EEG data [70]. |
| GAN Architectures (e.g., AnEEG, GCTNet) | Software/Algorithm | Generates artifact-free EEG signals through adversarial training, effectively handling non-linear and non-stationary noise [66]. |
| Public BCI Datasets (e.g., Mobile BCI [69], PhysioNet) | Data | Provides standardized, annotated data for training, validating, and benchmarking new artifact removal algorithms. |
The pursuit of higher SNR is a central and ongoing challenge in BCI research, driving innovation across multiple domains. As this guide has detailed, effective noise and artifact mitigation is no longer reliant on a single method but rather on a synergistic combination of strategies. These include designing robust paradigms, selecting appropriate signal acquisition technologies, and deploying advanced processing algorithms ranging from traditional ICA to sophisticated deep learning models like ART and GCTNet. The emergence of multi-modal approaches, which fuse EEG with data from sensors like IMUs, represents a particularly promising direction for tackling the formidable problem of motion artifacts in real-world BCI applications. As these techniques continue to mature, they will undeniably propel the field forward, enabling more reliable, efficient, and user-friendly BCI systems for clinical, assistive, and consumer applications.
The efficacy of any Brain-Computer Interface (BCI) is fundamentally constrained by the biological response it elicits upon implantation. The foreign body response triggers a cascade of events leading to chronic inflammation and the formation of an insulating glial scar around the implant. This scar tissue increases the distance between neurons and recording sites, causing rapid signal attenuation and a sharp rise in impedance, which ultimately degrades recording quality and stimulation efficiency, leading to device failure [72] [73]. The central challenge lies in the mechanical mismatch between traditional rigid electrode materials (e.g., silicon, platinum) and soft brain tissue, which has a Young's modulus of approximately 1–10 kPa [72]. This mismatch causes continuous micro-motions, damaging neurons and nerve fibers and sustaining the inflammatory cycle [73].
Quantitative Analysis of the Foreign Body Response
A detailed understanding of the tissue response is crucial for developing mitigation strategies. The table below summarizes key quantitative findings from in vivo studies on the tissue reaction to implanted neural interfaces.
Table 1: Quantitative Metrics of Foreign Body Response to Neural Implants
| Metric | Findings | Source/Model |
|---|---|---|
| Neuronal Density Reduction | Decreased to 24 ± 28% of control up to 20 μm from implant. 74 ± 39% of control at 20–40 μm. Control-like density beyond 40 μm [74]. | SU-8 implants in rat neocortex, 2-month survival [74]. |
| Astrogliosis Range | Significant increase in astroglial staining up to 560 μm (superficial layers) and 480 μm (deep layers) from implant track [74]. | SU-8 implants in rat neocortex, 2-month survival [74]. |
| Glial Scar Thickness | Electron microscopy revealed a thin glial scar, approximately 5–10 μm thick, surrounding the implant [74]. | SU-8 implants in rat neocortex [74]. |
| Synaptic Density Recovery | Density of synaptic contacts decreased near the implant but recovered to control levels at a distance of 24 μm from the implant track [74]. | SU-8 implants in rat neocortex, electron microscopy [74]. |
| Scar Tissue Impact | Collagen density (key scar component) reduction of up to 68% with new polymer designs, improving signal transport [75]. | Semiconducting polymer devices in mouse models [75]. |
Experimental Protocols for Assessing Biocompatibility
To evaluate the biocompatibility of new neural interfaces, standardized in vivo protocols and histological analyses are essential. The following methodology provides a framework for quantitative assessment.
Protocol: Immunohistochemical Quantification of Biocompatibility
This protocol is adapted from a detailed in vivo biocompatibility study [74].
- Objective: To quantitatively evaluate neuronal preservation, astrogliosis, and glial scar formation around an implanted neural sensor after a chronic implantation period.
Materials:
- Experimental animals (e.g., rat model).
- Neural implant device (e.g., SU-8 based).
- Surgical equipment for stereotactic implantation.
- Perfusion and fixation solutions (e.g., paraformaldehyde).
- Cryostat or microtome for sectioning.
- Primary antibodies: e.g., NeuN (for neurons), GFAP (for astrocytes).
- Secondary antibodies with fluorescent tags.
- Electron microscopy reagents (if analyzing ultrastructure).
Methodology:
- Implantation: Stereotactically implant the neural device into the target brain region (e.g., neocortex). Ensure all procedures adhere to ethical guidelines for animal research.
- Survival Period:
- Allow an appropriate survival period to assess chronic response (e.g., 2 months) [74].
- Tissue Preparation:
- Transcardially perfuse the animal with fixative.
- Extract the brain, post-fix, and cryoprotect.
- Section the tissue containing the implant track into coronal slices (e.g., 40 μm thickness).
- Immunohistochemical Staining:
- Perform immunofluorescence staining using antibodies against NeuN and GFAP.
- Use appropriate controls to validate staining specificity.
- Image Acquisition and Quantification:
- Acquire high-resolution images of the tissue surrounding the implant track using confocal or epifluorescence microscopy.
- Neuronal Density: Count NeuN-positive cells in concentric zones from the implant track (e.g., 0–20 μm, 20–40 μm, 40–100 μm) and normalize to a control region from the contralateral hemisphere [74].
- Astrogliosis Intensity: Measure the fluorescence intensity of GFAP staining in the same concentric zones.
- Ultrastructural Analysis (Optional):
- Process tissue for electron microscopy.
- Measure the thickness of the glial scar and quantify the density of synaptic contacts at various distances from the implant [74].
Signaling Pathways and Molecular Mechanisms
The foreign body response is a complex, multi-stage process. The following diagram illustrates the key signaling pathways and cellular events from implantation to chronic scar formation.
Diagram 1: Foreign Body Response to Neural Implants.
Strategies for Enhancing Long-Term Stability
Addressing the biocompatibility challenge requires a multi-faceted approach targeting material properties, device design, and active modulation of the immune response.
Material and Mechanical Compatibility
The primary goal is to minimize the mechanical mismatch at the tissue-electrode interface.
- Flexible Electronics: Shift from rigid substrates (silicon, ∼102 GPa) to flexible polymers (e.g., SU-8, polyimide) with a lower Young's modulus to better match brain tissue (1–10 kPa) [72] [73]. SU-8, for instance, demonstrates high biocompatibility, with neuronal effects confined to a very small area [74].
- Geometric Optimization: Reduce the cross-sectional area of electrodes to subcellular dimensions. Filamentary electrodes with widths as small as 7 μm and thicknesses of 1.5 μm can be guided by thin shuttles, drastically reducing acute injury and chronic inflammation [73].
- Implantation Method Coordination: The electrode shape must be matched with an appropriate implantation method.
- Unified Implantation: Uses a single rigid shuttle (e.g., tungsten wire) to deploy multiple electrodes simultaneously, ideal for deep brain detection [73].
- Distributed Implantation: Uses multiple independent guidance systems to deploy ultra-small electrodes sequentially, minimizing the cross-sectional area of each implantation and promoting better wound healing [73].
Surface Functionalization and Active Modulation
Beyond passive mechanical compatibility, active strategies aim to modulate the biological environment.
- Biocompatible Coatings: Apply coatings to suppress the immune response. Recent research demonstrates that incorporating selenophene into the polymer backbone and adding immunomodulating materials to side chains can reduce collagen density (scar tissue) by up to 68% in mouse models [75].
- Drug-Eluting Systems: Develop controlled-release systems from the electrode surface to deliver anti-inflammatory drugs or other substances that actively inhibit the inflammatory cascade and promote tissue repair [73].
Table 2: Electrode Design and Coating Strategies for Enhanced Biocompatibility
| Strategy Category | Specific Approach | Intended Function & Mechanism |
|---|---|---|
| Passive Mechanical | Flexible substrates (SU-8, Polyimide) [74] [73] | Reduce mechanical mismatch and micro-motions, minimizing chronic irritation. |
| Passive Mechanical | Ultra-small filamentary electrodes (e.g., 7 μm width) [73] | Minimize acute tissue displacement and damage during implantation. |
| Active Chemical | Selenophene-based polymer backbones [75] | Intrinsically suppress the foreign-body response through material chemistry. |
| Active Chemical | Immunomodulating side chains [75] | Actively interact with the immune system to reduce inflammatory signaling. |
| Active Pharmaceutical | Drug-controlled release systems [73] | Localized delivery of anti-inflammatories to quell the immune response post-implant. |
The Scientist's Toolkit: Research Reagent Solutions
The following table lists key materials and reagents used in the development and testing of biocompatible neural interfaces.
Table 3: Essential Research Reagents for Biocompatibility Studies
| Reagent / Material | Function in Research |
|---|---|
| SU-8 Polymer | A flexible, epoxy-based photoresist used as a substrate for fabricating neural probes. Its biocompatibility and mechanical properties are subjects of investigation [74]. |
| Selenophene-incorporated Polymers | Novel semiconducting polymers used in bioelectronics to suppress the foreign-body response by modulating the immune reaction at the material level [75]. |
| Anti-NeuN Antibody | A primary antibody used in immunohistochemistry to identify and quantify mature neuronal cells in tissue sections surrounding an implant [74]. |
| Anti-GFAP Antibody | A primary antibody used to label astrocytes. It is essential for quantifying the extent of astrogliosis (reactivity) in response to an implant [74]. |
| Polyethylene Glycol (PEG) | A biocompatible polymer used as a temporary coating to stiffen flexible electrodes for implantation. It dissolves after implantation, leaving the flexible electrode in place [73]. |
Brain-Computer Interfaces (BCIs) establish a direct communication pathway between the brain and external devices, offering transformative potential for restoring function in patients with neurological disorders and enhancing human-computer interaction [54] [1]. The core technical challenge lies in accurately interpreting intended user commands from complex, noisy neural signals. Electroencephalography (EEG) signals, particularly those associated with Motor Imagery (MI)—the mental rehearsal of physical actions—exhibit characteristics that complicate this task: they possess an inherently low signal-to-noise ratio (SNR) and are non-stationary and high-dimensional [76] [77].
Traditional signal processing and machine learning techniques often reach a performance plateau due to these challenges [77]. Consequently, the integration of Artificial Intelligence (AI), especially deep learning, has become pivotal in developing advanced processing pipelines. These AI-enhanced pipelines automate and optimize the stages of feature extraction and classification, leading to substantial improvements in the Intention Detection Rate (IDR) and the overall reliability of BCI systems [76] [11]. This technical guide examines the role of AI and deep learning within the core BCI workflow, providing a detailed analysis for researchers and scientists engaged in signal acquisition research.
The BCI Processing Pipeline: A Foundation for AI Integration
The standard BCI pipeline consists of four sequential stages: signal acquisition, preprocessing, feature extraction, and classification/translation. AI and deep learning are revolutionizing the latter three stages.
Table 1: Core Stages of the BCI Processing Pipeline
| Pipeline Stage | Core Function | Traditional Methods | AI/Deep Learning Enhancements |
|---|---|---|---|
| Signal Acquisition | Records neural activity from the brain [1]. | EEG, ECoG, intracortical microelectrode arrays [9]. | N/A (Hardware-dependent) |
| Preprocessing | Filters noise and artifacts to improve Signal-to-Noise Ratio (SNR) [76]. | Bandpass filters, Independent Component Analysis (ICA) [76]. | Hybrid decomposition techniques (e.g., EEMD + ICA), automated artifact detection [76]. |
| Feature Extraction | Identifies discriminative patterns in the cleaned signal [76]. | Band power, Common Spatial Patterns (CSP), wavelet transforms [76] [77]. | Automated learning of hierarchical features using CNNs; nonlinear features (e.g., fractal dimension) [76] [77]. |
| Classification | Translates features into device control commands [1]. | Support Vector Machines (SVM), Linear Discriminant Analysis (LDA) [76] [77]. | Convolutional Neural Networks (CNNs), Long Short-Term Memory (LSTM) networks, attention mechanisms [76] [77] [11]. |
The following diagram illustrates the complete BCI workflow, highlighting the integration of AI and deep learning components at each stage.
Diagram 1: The AI-enhanced BCI processing pipeline, demonstrating the closed-loop feedback system and AI integration points.
AI-Driven Preprocessing and Feature Extraction
Overcoming the Signal-to-Noise Ratio Challenge
The initial preprocessing stage is critical for enhancing SNR. Traditional linear approaches like Fast Fourier Transform (FFT) struggle with the nonlinear and non-stationary nature of EEG [76]. AI-informed decomposition techniques have emerged as superior alternatives:
- Empirical Mode Decomposition (EMD) and Variants: Methods like Ensemble EMD (EEMD) and Self-Adaptive Multivariate EMD (SA-MEMD) adaptively decompose signals into intrinsic mode functions, preserving time-frequency characteristics without assuming linearity [76].
- Hybrid Decomposition: Combining EMD with ICA or wavelet packet analysis effectively mitigates issues like mode mixing and improves artifact removal [76].
Automated and Nonlinear Feature Extraction
Moving beyond manually engineered features like band power, deep learning automates the discovery of optimal feature representations.
- Convolutional Neural Networks (CNNs) automatically learn spatially relevant features from raw EEG signals or their time-frequency representations (e.g., spectrograms) [77]. For instance, converting MI-EEG data into 2D time-frequency images via Short-Time Fourier Transform (STFT) allows CNNs to extract highly discriminative patterns [76].
- Nonlinear Feature Integration: Combining deep learning with nonlinear metrics such as fractal dimension (FD), sample entropy, and recurrence quantification captures the complex dynamics of brain activity. One study demonstrated that a multi-method FD combination classified MI tasks with 79.2% accuracy using a Linear SVM [76].
Table 2: AI and Deep Learning Models for Feature Extraction and Classification
| Model/Technique | Primary Function | Key Innovation | Reported Performance |
|---|---|---|---|
| Parallel CNNs (PCNNs) [76] | Feature Extraction & Classification | Extracts multi-scale features from EEG signals simultaneously. | Outperformed traditional baselines, though overfitting remains a challenge. |
| RP-BCNNs [76] | Classification | Combines Recurrence Plots (RP) with Bayesian CNNs to address variability. | 92.86% accuracy for real movements; 94.07% for imagined movements. |
| Attention-enhanced CNN-LSTM [77] | Classification | Integrates spatial (CNN), temporal (LSTM), and attention-based feature weighting. | 97.24% accuracy on a custom four-class MI dataset. |
| DDMSCNN with Attention [76] | Feature Extraction & Classification | Uses Double-Dimensional Multi-Scale CNN and attention on STFT-generated images. | 70.50% (user-dependent) and 64.04% (user-independent) accuracy on BCI Competition IV-2a. |
| Transfer Learning (TL) [11] | System Calibration | Reduces need for per-user calibration by leveraging data from other subjects. | Improves system adaptability and addresses inter-subject variability. |
Deep Learning Architectures for Classification
Advanced deep learning architectures have surpassed conventional classifiers like SVM and LDA by better modeling the spatiotemporal structure of EEG signals.
Hybrid Convolutional-Recurrent Networks
- Spatiotemporal Modeling: CNNs excel at extracting spatial features from multi-channel EEG data, mimicking hierarchical visual processing [77]. Subsequent Long Short-Term Memory (LSTM) layers model the temporal dynamics and oscillatory patterns of the extracted features [77] [11].
- Attention Mechanisms: Inspired by cognitive selective attention, these modules allow the model to dynamically focus on the most task-relevant spatial locations and temporal segments within the high-dimensional EEG data [77]. This "biomimetic" approach significantly enhances classification accuracy and provides interpretable insights into the neural signatures of MI.
Innovative Input Representations and Robust Models
- Recurrence Plots (RP) with Bayesian CNNs: Converting pre-processed EEG data into a Recurrence Plot, which visualizes the recurrence states of a dynamical system, and processing it with Bayesian CNNs has shown exceptional accuracy (~93-94%) for MI classification, effectively addressing inter-individual variability [76].
- The Closed-Loop Advantage: Modern BCI systems are increasingly closed-loop, meaning the output (e.g., movement of a robotic arm) provides real-time feedback to the user. This feedback allows users to adjust their mental strategy, and AI algorithms can adapt the decoding model in real-time, creating a synergistic cycle that enhances overall performance and facilitates neuroplasticity in therapeutic applications [54] [11].
The following diagram illustrates the architecture of a state-of-the-art hierarchical attention model that achieves high-precision classification.
Diagram 2: Hierarchical attention architecture for motor imagery classification.
Experimental Protocols and Research Toolkit
This section outlines a representative experimental protocol for training and evaluating a deep learning model on a public MI-EEG dataset, providing a reproducible methodology for researchers.
Detailed Experimental Protocol: MI-EEG Classification with an Attention-Enhanced CNN-LSTM
Dataset Selection:
- Dataset: BCI Competition IV-2a [76].
- Description: A standard public benchmark containing EEG data from 9 subjects performing 4-class MI tasks (left hand, right hand, feet, tongue). The dataset includes 4,320 trials [77].
- Preprocessing: Apply a bandpass filter (e.g., 4-40 Hz). Use techniques like Common Average Reference (CAR) or Automated Artifact Removal (e.g., Faster ICA) to remove ocular and muscular artifacts [76].
Data Preparation:
- Segmentation: Segment the continuous EEG into epochs time-locked to the MI cue presentation.
- Validation: Use a subject-specific k-fold cross-validation strategy to ensure robust performance estimation and mitigate overfitting.
- Input Formulation: For image-based models (e.g., CNNs), transform the raw EEG epochs into 2D representations using Short-Time Fourier Transform (STFT) or Continuous Wavelet Transform (CWT) to create time-frequency images [76].
Model Training:
- Architecture: Implement a hybrid CNN-LSTM model with an attention mechanism.
- Spatial Filtering: The CNN component (e.g., using parallel multi-scale kernels) processes the input to extract spatial features [76] [77].
- Temporal Modeling: The LSTM component processes the CNN's output sequences to capture temporal dependencies [77].
- Attention Layer: Integrate an attention layer to assign adaptive weights to the spatiotemporal features, allowing the model to focus on the most discriminative neural patterns [77].
- Hyperparameters: Optimize using a validation set. Key parameters include learning rate (e.g., 1e-4), batch size (e.g., 32), and the number of CNN filters and LSTM units.
Evaluation and Analysis:
- Metrics: Report standard performance metrics: Accuracy, Kappa coefficient, and F1-score.
- Ablation Study: Conduct an ablation study to quantify the individual contribution of the CNN, LSTM, and attention components to the overall performance [77].
- Visualization: Generate visualization plots (e.g., attention maps) to interpret which spatial regions and time points were most critical for the model's decision, enhancing explainability [77].
Table 3: Key Resources for BCI AI Research
| Resource Type | Specific Example | Function in Research |
|---|---|---|
| Public BCI Datasets | BCI Competition IV-2a, IV-2b; PhysioNet Database [76]. | Provides standardized, annotated EEG data for training, benchmarking, and comparing algorithms. |
| Signal Decomposition Algorithms | Self-Adaptive Multivariate EMD (SA-MEMD), Ensemble EMD (EEMD) [76]. | Preprocessing tools to handle non-stationarity and improve SNR before feature extraction. |
| Deep Learning Frameworks | TensorFlow, PyTorch. | Open-source libraries for building and training custom neural network models (e.g., CNNs, LSTMs). |
| Nonlinear Feature Metrics | Fractal Dimension (FD), Sample Entropy, Recurrence Quantification Analysis (RQA) [76]. | Provides quantitative descriptors of signal complexity that can be used as features or validation for deep learning models. |
| Classification Models | Support Vector Machines (SVM), CNNs, LSTMs, Attention Networks [76] [77] [11]. | Core algorithms for translating extracted features into device control commands. |
The integration of AI and deep learning into BCI processing pipelines has fundamentally advanced the field, moving it from reliance on handcrafted features to automated, hierarchical feature learning. Architectures that synergistically combine spatial convolutional filters, temporal recurrent networks, and attention-based selection have demonstrated remarkable performance, achieving classification accuracies exceeding 97% in controlled settings [77]. These advancements are directly translating into more robust and clinically viable BCI systems for neurorehabilitation, assistive communication, and the diagnosis of neurological disorders [54] [11] [16].
Despite significant progress, challenges remain. Data scarcity necessitates innovative solutions like transfer learning and data augmentation. Improving the interpretability of complex deep learning models is crucial for building trust, especially in clinical applications. Furthermore, achieving real-time processing with low latency and developing adaptive algorithms that can handle non-stationarity across sessions and subjects are critical areas for ongoing research [76] [11]. Future work will focus on refining these AI models, enhancing their efficiency and generalizability, and ultimately paving the way for the widespread clinical adoption and commercialization of BCI technology, potentially restoring function and improving the quality of life for millions affected by neurological conditions [1] [9] [16].
Optimizing User Training and Adaptive Algorithms for Enhanced BCI Performance
Brain-Computer Interface (BCI) technology establishes a direct communication pathway between the brain and external devices, offering transformative potential in both clinical and non-clinical domains [4]. The efficacy of a BCI system hinges on a critical interplay between two adaptive components: the human user's ability to generate consistent, discriminative brain signals, and the computational system's capacity to accurately decode these non-stationary neural patterns [78]. Despite promising laboratory demonstrations, achieving reliable BCI performance in real-world conditions remains a significant hurdle, often due to the unstable nature of electroencephalography (EEG) signals and the variability in user skill [78] [79].
This technical guide addresses the core challenge of enhancing BCI performance through a dual-pronged approach: optimizing user training protocols to improve the quality of signal generation and implementing adaptive algorithms that evolve with the user's changing brain signals. The pursuit of this optimization is not merely academic; it is essential for transitioning BCIs from controlled laboratory settings to practical, out-of-the-lab applications, thereby fulfilling their promise as robust assistive technologies [78] [80].
BCI Working Principles and Signal Acquisition Framework
Core BCI Architecture
At its foundation, a BCI is a closed-loop system comprising four integral components that work in concert: signal acquisition, processing, output, and feedback [4]. The process begins with signal acquisition, where sensors capture neural activity. These signals are then processed through a series of steps including preprocessing (e.g., noise filtration) and decoding (often using machine learning algorithms) to interpret the user's intent. The processed intent is translated into an output command to control an external device, such as a robotic arm or a virtual car. Finally, the system provides feedback to the user about the action taken, enabling a closed-loop design where the user can adjust their mental strategy accordingly [4] [1]. This continuous loop is the backbone of effective BCI operation.
A Two-Dimensional Framework for Signal Acquisition
The initial signal acquisition stage is paramount, as it determines the theoretical upper limit of system performance. Recent research proposes a comprehensive two-dimensional framework to categorize and evaluate signal acquisition technologies, synthesizing both clinical and engineering perspectives [4] [6].
Table 1: Two-Dimensional Framework for BCI Signal Acquisition Technologies
| Dimension | Perspective | Categories & Definitions | Impact on Performance |
|---|---|---|---|
| Surgery (Invasiveness) [4] | Clinical | Non-invasive: No anatomical trauma (e.g., EEG cap).Minimally-invasive: Trauma sparing brain tissue (e.g., endovascular stent).Invasive: Trauma affecting brain tissue (e.g., implanted electrode array). | Increasing invasiveness generally offers higher signal fidelity but carries greater surgical risk, ethical considerations, and clinical implementation challenges. |
| Detection (Sensor Location) [4] | Engineering | Non-implantation: Sensor on body surface.Intervention: Sensor within a natural body cavity (e.g., blood vessel).Implantation: Sensor within human tissue. | Determines the proximity to the neural signal source, directly influencing the theoretical upper limit of signal quality and long-term biocompatibility. |
This framework clarifies the fundamental trade-off in BCI design: as one moves from non-invasive to invasive approaches, and from non-implantation to implantation, the potential signal quality improves, but this comes at the cost of increased medical risk and complexity [4]. For instance, non-invasive EEG, while safe and accessible, suffers from attenuated signals due to the skull and scalp, whereas invasive technologies like the Utah array can record from individual neurons but require craniotomy and carry infection risks [1] [80]. Emerging minimally-invasive technologies, such as Synchron's Stentrode (endovascular) and Precision Neuroscience's Layer 7 (epicortical), aim to strike a balance by offering higher-quality signals without penetrating brain tissue [1] [80].
Diagram 1: BCI architecture and signal acquisition framework.
User Training Protocols for BCI Skill Acquisition
The human user is an integral, adaptive component of the BCI loop. Effective training is therefore required to teach users to self-regulate their brain activity and produce stable, distinct neural patterns.
Progressive Training Methodology
A longitudinal study involving a tetraplegic user training for the CYBATHLON BCI competition over 20 sessions demonstrated the efficacy of a structured, progressive training regimen [78]. The protocol was divided into distinct phases to gradually build user competency, as Artificer's study showed a 30% improvement in classification accuracy by the end of the training compared to initial sessions [78].
Table 2: Progressive BCI User Training Protocol (20 Sessions)
| Training Phase | Sessions | Primary Focus | Mental Tasks / Paradigm | Key Metrics & Outcomes |
|---|---|---|---|---|
| Exploratory & Calibration | 1-7 | Identifying optimal mental tasks and EEG sensor setup. | Testing of various cognitive (e.g., mental subtraction) and motor imagery (MI) tasks (e.g., left/right hand). | Selection of the most distinct and reliable mental commands for the user. |
| Focused 2-Class Training | 8-11 | Mastering control of a basic 2-class BCI system. | Practicing two selected tasks (e.g., REST vs. MENTAL SUBTRACTION or LEFT-HAND vs. RIGHT-HAND MI). | Building foundational skills for generating discriminative EEG patterns in a closed-loop setting. |
| Advanced 4-Class & Game Transfer | 12-20 | Integrating control and applying skills in the target environment. | Combining all 4 mental tasks. Transitioning to practice with the actual racing game environment. | Improvement in synchronous (cue-based) BCI performance; translation of skills to a dynamic application. |
Data Visualization as Feedback
Beyond conventional feedback that merely indicates success or failure, advanced protocols provide users with intuitive visualizations of their brain activity. One study proposed an online data visualization feedback protocol that represents EEG trial distribution in Riemannian geometry in real-time [81]. In this paradigm, subjects learn to modulate their sensorimotor rhythm to centralize data points within the same category and separate points from different categories on a screen. This method provides a more nuanced and informative feedback mechanism, helping users understand and improve their mental command control without relying on a pre-trained classifier [81].
Adaptive Algorithms for Handling Non-Stationarity
The EEG signals produced by the brain are inherently non-stationary, meaning their statistical properties change over time due to factors like fatigue, attention shifts, and learning. This poses a major challenge for static machine learning classifiers. Adaptive algorithms are therefore essential for maintaining robust BCI performance.
Riemannian Geometry-Based Classification
A powerful modern approach in BCI signal processing leverages Riemannian geometry [78] [81]. This method involves representing EEG trials as spatial covariance matrices and analyzing them on a Riemannian manifold. The strength of this approach lies in its robustness to noise and outliers, and its ability to achieve high performance without extensive calibration or complex spatial filtering optimization [78]. A key advantage for longitudinal use is that Riemannian classifiers can be adapted to model both within-session and between-session variabilities. For instance, between-session non-stationarity can be mitigated by projecting data from all sessions to a common reference using the geometric mean of a few minutes of initial EEG data from each new session [78].
Error-Related Potential (ErrP) Detection for Self-Correction
Another sophisticated adaptive strategy incorporates the detection of error-related potentials (ErrPs) [79]. ErrPs are naturally occurring event-related potentials that are elicited when a user perceives an error, such as a misclassification by the BCI system. An online neurofeedback closed-loop system can be designed to detect these ErrPs and use them to correct the BCI's output automatically [79].
The workflow of such a system is as follows:
- The BCI decodes the user's intended command (e.g., from motor imagery).
- The system executes the command and provides feedback.
- If the user perceives an error, their brain generates an ErrP.
- The system detects this ErrP in real-time.
- Upon ErrP detection, the system cancels or corrects the erroneous command.
- Crucially, the correctly-labeled data (the original EEG trial and the corrected label) are then added to the training set, allowing the classifier to continuously update and improve without requiring explicit, lengthy recalibration sessions [79].
This ErrP-based adaptive system creates a smarter, more collaborative interaction between the user and the BCI, moving towards a system that learns and adapts with the user.
Diagram 2: Adaptive BCI loop with ErrP-based correction.
The Scientist's Toolkit: Essential Research Reagents & Materials
To implement and study the training protocols and algorithms discussed, researchers rely on a suite of specialized tools, software, and hardware.
Table 3: Essential Research Toolkit for BCI Optimization Studies
| Tool / Reagent | Category | Primary Function | Example Use Case / Note |
|---|---|---|---|
| High-Density EEG System | Hardware | Acquires scalp-level neural signals with high temporal resolution. | Foundation for non-invasive BCI studies; used in Motor Imagery (MI) and ErrP paradigms [78] [79]. |
| Implantable Electrode Arrays (e.g., Utah Array, Stentrode) | Hardware | Provides high-fidelity neural recording from the cortical surface or within tissue. | Used in invasive and minimally-invasive BCIs for superior signal quality [1] [80]. |
| OpenViBE | Software | An open-source platform for designing, testing, and running real-time BCI experiments. | Used for online signal acquisition, processing, and providing feedback in MI-BCI protocols [82]. |
| Riemannian Geometry Classification (e.g., PyRiemann) | Algorithm | A robust framework for classifying EEG trials based on covariance matrices on a manifold. | Core algorithm for handling non-stationarity; used in adaptive classification pipelines [78] [81]. |
| Channel-Weighted CSP (CWCSP) | Algorithm | A variant of Common Spatial Patterns that weights channels by contribution, reducing noise. | Feature extraction algorithm used in conjunction with ErrP detection for improved decoding accuracy [79]. |
| ErrP Dataset | Data | A collection of EEG recordings from subjects observing errors, used to train ErrP detectors. | Enables the development of ErrP-based correction systems; classifiers can be transferable across user groups [79]. |
Optimizing BCI performance is a multifaceted challenge that requires simultaneous advancement in user training and machine learning adaptability. The path forward lies in developing integrated systems that not only train users to produce more stable brain signals through progressive, informative protocols but also deploy intelligent adaptive algorithms, such as those based on Riemannian geometry and error-potential detection, to form a truly collaborative and resilient human-machine partnership. As signal acquisition technologies continue to evolve, providing a richer stream of neural data, the sophistication and reliability of the user training and adaptive algorithms described in this guide will become ever more critical in unlocking the full potential of BCI systems.
Benchmarking BCI Performance and Evaluating Commercial & Research Platforms
In brain-computer interface (BCI) research, the performance and applicability of a system are fundamentally governed by three core technical metrics: spatial resolution, temporal resolution, and accuracy, which collectively determine the information transfer rate (ITR) or bandwidth [83]. These metrics are not independent; they are intrinsically linked to the chosen signal acquisition methodology, which ranges from non-invasive scalp recordings to fully implanted micro-electrodes [6] [4] [23].
The pursuit of higher performance in BCIs involves navigating critical trade-offs, primarily centered on the invasiveness of the technology [1] [4]. This whitepaper provides an in-depth analysis of these key performance metrics, detailing their definitions, the methodologies for their measurement, and their interdependence. It is structured to serve as a technical reference for researchers and developers working on the signal acquisition pillar of BCI systems, framing the discussion within the broader context of BCI working principles and the escalating demand for robust, high-fidelity neural interfaces.
Core Performance Metrics in BCI Signal Acquisition
The efficacy of a BCI system is predominantly contingent upon its signal acquisition module [6] [4] [23]. The following metrics define its capabilities and limitations.
Spatial Resolution
Spatial resolution refers to the ability of a BCI system to distinguish between distinct neural activity sources within the brain. It is a key differentiator between acquisition technologies.
- Definition: The granularity with which a system can localize the origin of neural signals, typically measured in millimeters or centimeters for non-invasive methods, and micrometers for invasive arrays [83].
- Dependence: This metric is primarily determined by the physical distance between the signal source (neurons) and the sensor, as well as the density and configuration of the recording electrodes [4]. As an analogy, detecting brain activity with a non-implantation method is "like listening from outside the building, where only a large-scale sum of neuronal activity can be heard" [4].
- Impact: Higher spatial resolution enables more precise decoding of intent, such as differentiating between individual finger movements or decoding articulate speech patterns [1].
Temporal Resolution
Temporal resolution describes the system's precision in tracking changes in neural activity over time.
- Definition: The smallest time interval of neural activity that a BCI system can resolve, effectively the sampling rate of the system, measured in Hertz (Hz) [83].
- Typical Range: Most BCI systems operate within a 0.1-100 Hz frequency range to capture relevant brain rhythms, from slow delta waves to fast gamma oscillations [83]. The raw analog signals are digitized at sampling rates between 250-10,000 Hz to accurately represent these frequencies [83].
- Impact: High temporal resolution is crucial for capturing rapid neural dynamics, such as the timing of action potentials or the precise onset of an event-related potential like the P300 [23].
Accuracy and Information Transfer Rate (ITR)
While spatial and resolution metrics describe input quality, accuracy and ITR quantify the system's output performance.
- Accuracy: The success rate with which a user's intended command is correctly decoded and executed by the BCI system. Modern systems, particularly those using deep learning, have achieved decoding accuracies exceeding 99% for tasks like speech inference with latencies under <0.25 seconds in research settings [1].
- Information Transfer Rate (ITR): A composite metric, often measured in bits per minute, that reflects the overall communication speed of a BCI. It incorporates both accuracy and the number of potential targets. Non-invasive BCIs typically achieve ITRs of 5-25 bits/minute, while invasive BCIs can reach over 200 bits/minute [83].
Table 1: Key Performance Metrics Across BCI Signal Acquisition Modalities
| Acquisition Method | Spatial Resolution | Temporal Resolution | Typical Accuracy/ITR | Primary Trade-offs |
|---|---|---|---|---|
| Electroencephalography (EEG) [83] | Low (centimeters) | High (milliseconds) | ITR: 5-25 bits/minute [83] | Non-invasive, but low signal-to-noise ratio and susceptibility to artifacts |
| Electrocorticography (ECoG) [83] | Medium (millimeters) | High (milliseconds) | Higher than EEG; enables basic control and communication [1] [83] | Requires craniotomy; higher signal quality than EEG but less than intracortical arrays |
| Microelectrode Arrays (e.g., Utah Array, Neuralace) [1] [83] | High (micrometers) | High (milliseconds) | ITR: Up to 200+ bits/minute; high accuracy for complex tasks [1] [83] | Highest signal fidelity but risk of tissue scarring and signal degradation over time |
| Endovascular Stentrode (Synchron) [1] | Medium (millimeters) | High (milliseconds) | Allows control of digital devices for texting, etc. [1] | Minimally invasive via blood vessels; avoids brain tissue damage but signals recorded through vessel wall |
Experimental Protocols for Metric Validation
Robust experimental protocols are essential for the empirical validation of the performance metrics described above. The following outlines a standardized methodology for a closed-loop BCI experiment, which is critical for assessing real-world system performance.
Protocol: Closed-Loop BCI Performance Assessment
Objective: To quantitatively evaluate the spatial/temporal resolution, decoding accuracy, and Information Transfer Rate (ITR) of a BCI system under a specific paradigm (e.g., Motor Imagery or SSVEP) [67] [23] [13].
Workflow Description: The experimental process begins with Subject Recruitment & Setup, where participants are fitted with the signal acquisition device (e.g., EEG cap or implant) [13]. The Signal Processing Pipeline involves acquiring raw neural data, which is then amplified and digitized. The data undergoes preprocessing including filtering (e.g., bandpass 0.1-100 Hz, notch at 50/60 Hz) and artifact removal to improve the signal-to-noise ratio (SNR) [83]. Subsequently, Feature Extraction identifies discriminative patterns in the signal, such as power in specific frequency bands for Motor Imagery or evoked responses for P300 [67] [23].
In the AI Decoding & Execution stage, machine learning models (e.g., Support Vector Machines, Convolutional Neural Networks) are trained and validated to classify the features into user intents [67] [13]. During Closed-Loop Testing, the real-time decoded commands control an external device, and the system provides immediate Visual/Auditory Feedback to the user, creating an adaptive loop [67] [23]. Finally, Performance Metric Calculation involves computing accuracy, latency, and ITR based on the system's outputs during the testing phase [83].
Key Parameters to Record:
- Spatial Resolution: Determined by the acquisition hardware (e.g., number and placement of electrodes) [4] [83].
- Temporal Resolution: Defined by the sampling rate (e.g., 250-10,000 Hz) and system latency [83].
- Trial Count and Duration: Essential for statistical power and ITR calculation.
- Signal-to-Noise Ratio (SNR): A critical factor influencing all other performance metrics [67].
The Scientist's Toolkit: Research Reagent Solutions
The advancement of BCI performance is enabled by a suite of specialized materials, hardware, and algorithms. The following table details key components of the modern BCI research toolkit.
Table 2: Essential Research Tools for High-Performance BCI Development
| Tool Category | Specific Examples | Function & Importance in Performance |
|---|---|---|
| Electrode Materials [83] | - Microelectrode arrays (Silicon, Platinum, Iridium Oxide)- Flexible substrates (Polyimide, Parylene-C)- Conductive coatings (PEDOT:PSS, Carbon Nanotubes) | Determine biocompatibility, long-term signal stability, and impedance. Flexible materials reduce immune response, preserving high spatial resolution over time. |
| Signal Acquisition Hardware [83] | - High-resolution Amplifiers- Analog-to-Digital Converters (ADC)- Wireless Transmitters (e.g., Bluetooth) | Amplifiers boost microvolt-level signals. ADC resolution (12-24 bit) and sampling rate (up to 10k Hz) define the system's temporal resolution and dynamic range. |
| AI/ML Decoding Algorithms [67] [13] | - Convolutional Neural Networks (CNN)- Support Vector Machines (SVM)- Canonical Correlation Analysis (CCA) | Directly impact decoding accuracy and latency. Deep learning models (e.g., CNN) enable agnostic decoding of complex patterns, boosting ITR. |
| Stimulation & Paradigm Tools [23] [13] | - Visual Stimulators (LEDs for SSVEP)- Motor Imagery Paradigms- P300 Spellers | Elicit robust and classifiable neural responses. The design of the paradigm is crucial for generating signals with high SNR and separability. |
Signaling Pathways and System Integration
A BCI system functions as an integrated signal processing chain, where performance at each stage dictates the overall system capability. The relationship between the core metrics and the system's functional pathway can be visualized as follows.
Pathway Description: The process initiates with Neural Signal Generation from individual neurons and neural populations. The Signal Acquisition stage, utilizing technologies like EEG, ECoG, or intracortical microelectrodes, is the primary determinant of the system's maximum achievable spatial and temporal resolution [4] [83]. The acquired signal then undergoes Preprocessing & Feature Extraction, where noise is filtered and discriminative features are enhanced. The AI/ML Decoding Algorithm translates these features into control commands, a stage where the fidelity of the preceding steps culminates in the final Accuracy & ITR of the system [67]. The resulting Device Output & Feedback completes the closed-loop system, which is essential for user adaptation and effective control [67] [23].
The relentless advancement of BCI technology is a direct function of improvements in its core performance metrics: spatial resolution, temporal resolution, and accuracy. As this analysis demonstrates, these metrics are deeply interconnected and fundamentally constrained by the signal acquisition methodology, creating a persistent trade-off between invasiveness and performance [6] [4]. Future progress hinges on interdisciplinary collaboration between clinicians and engineers to develop next-generation solutions—such as advanced biocompatible materials, sophisticated AI decoding algorithms, and minimally invasive surgical techniques—that successfully balance signal fidelity with safety and practicality [6] [67]. The standardization of performance validation protocols, as outlined herein, will be crucial for objectively comparing these emerging technologies and propelling the entire field toward more effective, reliable, and clinically viable brain-computer interfaces.
A Brain-Computer Interface (BCI) is a system that creates a direct communication pathway between the brain and an external device [1]. The core function of any BCI is to translate thought into action through a standardized processing pipeline, regardless of its specific design [11] [1]. This pipeline begins with signal acquisition, where sensors detect neurophysiological signals [6]. The raw signals then undergo preprocessing and feature extraction to filter noise and identify meaningful patterns [11]. Next, a decoding algorithm translates these features into commands, which are finally executed as an output to control an external device, such as a computer cursor or robotic arm [1]. Most advanced systems operate as closed-loop systems, where the user receives feedback on the action, allowing them to adjust their mental strategy in real time, creating an adaptive interface [11] [1].
The following diagram illustrates this universal BCI workflow.
Core Divergence: Invasive vs. Non-Invasive Signal Acquisition
The most fundamental distinction in BCI technology lies in the method of signal acquisition, which creates a trade-off between signal fidelity and practicality/risk [84] [32].
- Invasive BCIs are implanted inside the skull, either on the surface of the brain (electrocorticography, ECoG) or within the brain tissue (intracortical). This direct contact provides access to high-frequency neural signals with excellent spatial and temporal resolution [84]. However, it requires surgery, which carries risks of infection, tissue scarring, and immune response [84] [1].
- Non-Invasive BCIs measure brain activity from outside the skull. The most common method is electroencephalography (EEG), which uses electrodes on a headcap to record electrical activity [85] [32]. Other methods include functional near-infrared spectroscopy (fNIRS), which measures blood flow changes, and magnetoencephalography (MEG), which detects magnetic fields induced by neural activity [84] [86]. These methods are safe and accessible but suffer from the signal being blurred and attenuated by the skull and scalp, resulting in lower spatial resolution and bandwidth [84] [32].
The table below summarizes the key characteristics of different signal acquisition modalities.
Table 1: Comparison of BCI Signal Acquisition Technologies
| Feature | Invasive (Intracortical, e.g., Neuralink) | Invasive (ECoG, e.g., Precision) | Minimally Invasive (Endovascular, e.g., Synchron) | Non-Invasive (EEG) | Non-Invasive (fNIRS) |
|---|---|---|---|---|---|
| Spatial Resolution | Very High (single neuron) [84] | High (neuronal population) [1] | Moderate [84] | Low [84] | Moderate [86] |
| Temporal Resolution | Very High (kHz) [84] | Very High (kHz) [1] | High [84] | High (ms) [32] | Low (seconds) [86] |
| Invasiveness/Risk | High (craniotomy) [84] | Moderate (dura incision) [1] | Low (endovascular) [84] | None [85] | None [86] |
| Signal Bandwidth | Very High [84] | High [1] | Moderate-High [84] | Low [32] | Low [86] |
| Primary Signal | Neural spiking activity [84] | Local field potentials [1] | Local field potentials [84] | Scalp potentials [32] | Hemodynamic response [86] |
Technical Deep Dive: Leading Platforms and Methodologies
Invasive Platform Analysis
Neuralink Neuralink employs an array of ultra-thin, flexible polymer threads, each containing multiple electrodes, which are implanted into the cerebral cortex by a specialized robotic surgeon (R1) [87] [1]. The N1 implant is sealed within the skull and transmits data wirelessly. Its high channel count aims to record from large populations of neurons. A key recent experiment involves the "Telepathy" human trial. The experimental protocol for a typical session involves:
- Calibration: The patient performs imagined motor tasks (e.g., moving a cursor) while the system records neural patterns to build a decoder model [87].
- Real-time Control: The patient uses the trained model to control a computer interface.
- Adaptive Recalibration: The model degrades over time, requiring frequent recalibration sessions, which historically could take up to 45 minutes. A major software focus for Neuralink is reducing this to a few minutes [87].
Blackrock Neurotech Blackrock's established technology is based on the Utah Array, a bed of 100 rigid silicon microneedles [84]. A significant limitation is its poor "butcher ratio"—the number of neurons killed versus the number recorded from is highly unfavorable, leading to scarring and signal degradation over time [84]. The company is developing Neuralace, a flexible lattice designed for broader cortical coverage with less tissue damage [1]. A standard experimental protocol using the Utah Array for communication involves:
- Implantation: A craniotomy is performed to implant the array into the motor cortex [84].
- Signal Recording: Patients imagine limb movements, and the system records the corresponding motor cortex activity.
- Decoder Training: Machine learning algorithms map specific neural firing patterns to intended cursor movements or clicks.
- Output: Patients have achieved typing speeds of up to 90 characters per minute using this direct neural control [88].
Non-Invasive Platform Analysis
The non-invasive landscape is dominated by EEG-based systems like Kernel Flow, which uses high-density fNIRS to measure hemodynamic responses [88]. The core methodology for a non-invasive BCI intervention, particularly in neurorehabilitation for conditions like spinal cord injury, follows a structured protocol [85]:
- Setup: An EEG cap is positioned according to the international 10-20 system, and conductive gel is applied to ensure good signal quality [32].
- Paradigm Selection: The patient is presented with a specific task, such as motor imagery (e.g., imagining hand movement without physically moving it).
- Signal Acquisition & Processing: EEG signals are acquired, then heavily filtered to remove artifacts from eye blinks, muscle movement, and ambient noise [11] [32].
- Closed-Loop Feedback: The processed signal is classified in real-time (e.g., using a Support Vector Machine) to detect the intent of movement, which triggers feedback, such as moving a virtual hand or activating functional electrical stimulation (FES) on the patient's paralyzed limb [85] [11].
- Assessment: Functional outcomes are measured using standardized scales like the ASIA (American Spinal Injury Association) motor score or the Spinal Cord Independence Measure (SCIM) [85].
The divergent pathways for invasive and non-invasive BCIs are summarized in the diagram below.
Quantitative Performance and Market Outlook
Table 2: Comparative Performance and Commercial Metrics of Leading BCI Platforms
| Metric | Neuralink | Blackrock Neurotech | Synchron | Non-Invasive BCI (EEG/fNIRS) |
|---|---|---|---|---|
| Key Clinical Outcome | >9 bits/sec cursor control [87] | ~90 chars/min typing [88] | Texting, web browsing [84] | Improved motor/sensory scores in SCI [85] |
| Regulatory Status (2025) | Early human trials [87] | Multiple human implants; pursuing FDA [88] | FDA clearance for early feasibility [84] | FDA-cleared devices for wellness/research [86] |
| Funding / Market | ~$650M raised [88] | Acquired by Tether ($200M) [84] | >$100M raised [84] | Market to grow to >$1.5B by 2035 [88] |
| Primary Application | Communication, motor restoration [87] | Communication, robotic control [88] | Communication [84] | Rehabilitation, cognitive monitoring [85] [11] |
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Research Reagents and Materials for BCI Experiments
| Item / Solution | Function in BCI Research |
|---|---|
| Microelectrode Arrays | Signal acquisition from neuron populations. Includes Utah Array (Blackrock) and flexible threads (Neuralink) [84] [1]. |
| Electrode Gel (Conductive Gel) | Essential for non-invasive EEG to reduce impedance between scalp and electrodes, improving signal-to-noise ratio [32]. |
| fNIRS Photodiodes & Lasers | Emit and detect near-infrared light to measure cerebral blood flow, as used in systems like Kernel Flow [88] [86]. |
| Biocompatible Encapsulants | Materials used to hermetically seal implantable devices, protecting electronics from the hostile biological environment [84]. |
| Data Acquisition (DAQ) System | Hardware for amplifying, filtering, and digitizing analog neural signals from electrodes or sensors [86] [1]. |
| Robotic Surgical System (R1) | Enables precise, minimally invasive implantation of ultra-fine electrode threads into cortical tissue [87]. |
The choice between invasive and non-invasive BCI platforms involves a direct trade-off between performance and practicality. Invasive interfaces from leaders like Neuralink and Blackrock Neurotech offer unparalleled bandwidth and control for restoring critical functions in severe paralysis, but they come with significant surgical risks and long-term biological challenges [84] [87]. Non-invasive platforms, while safer and more accessible, are limited by signal quality and are currently more suited for rehabilitation and monitoring applications [85] [32]. The future of BCI will likely be shaped by advancements that blur this dichotomy, such as minimally invasive endovascular (Synchron) and epidermal (Precision Neuroscience) technologies, alongside AI-driven signal processing that pushes the performance ceiling of non-invasive methods [84] [11] [1].
Brain-Computer Interface (BCI) technology represents a burgeoning interdisciplinary domain that facilitates direct communication between the brain and external devices. The efficacy of BCI systems is largely contingent upon progress in signal acquisition methodologies, which form the critical foundation for all subsequent neural decoding and application [4]. Current BCI development is characterized by a fundamental trade-off: the relationship between signal fidelity (quality and richness of neural data) and procedural invasiveness (the degree of surgical intervention required) [4] [89].
From a technical perspective, BCI systems can be conceptualized through a four-component framework: (1) Signal Acquisition—the detection and recording of cerebral signals; (2) Processing—analysis of recorded brain activity using specialized algorithms to interpret intended actions; (3) Output—execution of the intended action through devices like robotic arms or speech synthesizers; and (4) Feedback—informing the user of the system's interpretation to allow for adjustments in a closed-loop design [4]. The signal acquisition module bears the critical responsibility for initial detection and recording, making it the determinative factor for overall system performance.
This whitepaper examines three leading companies—Synchron, Paradromics, and Precision Neuroscience—through the dual lenses of surgical invasiveness and detection methodology, providing researchers with a technical analysis of their distinctive approaches to overcoming the fundamental challenges in BCI signal acquisition.
Technical Analysis of Leading BCI Companies
Paradromics Inc.
Core Technology: The Connexus BCI is a fully implantable, high-data-rate interface designed for long-term use. Its micro-electrodes, each thinner than a human hair, capture brain activity from individual neurons. The system utilizes materials widely trusted in medical implants (titanium and platinum-iridium) for biocompatibility and long-term stability. Signals travel from the brain to a compact receiver implanted in the chest, which wirelessly transmits data through the skin for processing by external artificial intelligence systems [90].
Technical Specifications and Status: Paradromics has demonstrated an industry-leading 200+ bits per second rate of information transfer in pre-clinical models. The company received FDA Investigational Device Exemption (IDE) approval in late 2025 for its Connect-One Early Feasibility Study, marking the first IDE approval for speech restoration with a fully implantable BCI. The study will initially enroll two participants with impaired speech and limited extremity movement due to severe loss of voluntary motor control [90] [91].
Commercial and Research Position: With strategic investment from NEOM Investment Fund, Paradromics is establishing a Brain-Computer Interface Center of Excellence within NEOM to spearhead clinical research and serve as a premier center for BCI-based healthcare in the MENA region. The company's initial application focuses on restoring independent communication for individuals with spinal cord injuries, stroke, or ALS, with future applications planned for mental health conditions including mood disorders and chronic pain [92].
Precision Neuroscience
Core Technology: The Layer 7 Cortical Interface is an ultra-thin cortical electrode array featuring 1,024 electrodes embedded in a flexible film that conforms to the brain surface. At one-fifth the thickness of a human hair, the device employs a non-penetrating design aimed at safer and more scalable deployment. The company received FDA clearance in April 2025 for commercial use with implantation durations of up to 30 days, representing the first full regulatory clearance granted to a company developing a next-generation wireless BCI [93] [94].
Technical Specifications and Status: Precision's technology is characterized by its minimal invasiveness and focus on restorating independence to people living with paralysis and other neurological conditions. The company has secured significant venture funding, including investment from SCI Ventures, a specialist venture fund focused on paralysis that is backed by leading foundations including the Christopher & Dana Reeve Foundation, Wings for Life, and Spinal Research [93] [94].
Commercial and Research Position: Precision stands out for its emphasis on less invasive solutions and its progress in translating BCI technology from laboratory research to clinical application. The company is positioned to address a significant market, with spinal cord injury affecting more than 20 million people worldwide and lifetime care costs that can exceed $6 million per individual in the United States [94].
Synchron Inc.
Core Technology: The Stentrode BCI platform is an endovascular brain-computer interface that translates brain activity into digital commands without open-brain surgery. The device is placed via a nonsurgical catheter procedure and interfaces with the motor cortex through the blood vessels. Neural signals generated from this interface enable hands-free control of digital devices through Bluetooth technology that connects brain activity directly to Apple-brand electronics [95].
Technical Specifications and Status: Synchron's first-generation Stentrode device features 16 electrodes, significantly fewer than some competitors, but has demonstrated capability in enabling severely paralyzed people to control personal devices. To date, Stentrode BCIs have been implanted in 10 patients with paralysis during clinical trials conducted in the U.S. and Australia. The company recently raised $200 million in financing to advance commercialization and develop a next-generation interface [95] [89].
Commercial and Research Position: Synchron's approach represents a strategic compromise between invasiveness and signal quality, offering a clinically viable pathway with lower procedural risk. The company is growing an AI team in New York City focused on training models to learn from brain data with the goal of decoding thought in real time, while a new engineering hub in San Diego is working to build the company's next-generation brain interface [89].
Table 1: Comparative Technical Specifications of Leading BCI Technologies
| Parameter | Paradromics | Precision Neuroscience | Synchron |
|---|---|---|---|
| Product Name | Connexus BCI | Layer 7 Cortical Interface | Stentrode |
| Implantation Approach | Fully implantable | Cortical surface placement | Endovascular (via blood vessels) |
| Electrode Count | Not specified (High-density) | 1,024 | 16 |
| Key Differentiator | High data-rate (200+ bits/sec) | Non-penetrating design; FDA cleared | No open-brain surgery required |
| Regulatory Status | FDA IDE approved (2025) | FDA cleared (April 2025) | Clinical trials stage |
| Primary Application | Speech restoration, computer control | Digital device control for paralysis | Hands-free digital device control |
Table 2: Signal Acquisition Characteristics Based on Surgical and Detection Dimensions [4]
| Company | Surgery Dimension | Detection Dimension | Theoretical Signal Quality | Clinical Implementation Complexity |
|---|---|---|---|---|
| Paradromics | Invasive | Implantation | High (direct neural recording) | High (requires neurosurgeon) |
| Precision Neuroscience | Minimally invasive | Implantation | Medium-High (cortical surface) | Medium (requires surgical expertise) |
| Synchron | Minimally invasive | Intervention | Medium (through blood vessels) | Medium (requires interventional expertise) |
BCI Signal Acquisition Framework
Two-Dimensional Classification System
A comprehensive framework for understanding BCI signal acquisition technologies requires synthesis of both clinical and engineering perspectives. Recent research proposes a two-dimensional model that classifies technologies based on: (1) Surgery Dimension—the invasiveness of the procedure, and (2) Detection Dimension—the operating location of sensors [4].
The Surgery Dimension encompasses three distinct levels:
- Non-invasive: Procedures that do not induce anatomically discernible trauma to the subject.
- Minimally invasive: Procedures that cause anatomical trauma but spare the brain tissue from impact.
- Invasive: Procedures that cause anatomically discernible trauma at the micron scale or larger, specifically affecting brain tissue [4].
The Detection Dimension classifies based on sensor location during operation:
- Non-implantation: Signals acquired through sensors on the surface of the body.
- Intervention: Sensors leverage naturally existing cavities within the human body (e.g., blood vessels) without harming original tissue integrity.
- Implantation: Signals collected from sensors implanted within human tissue [4].
This framework explains the fundamental trade-offs in BCI design. As systems move toward more invasive surgical approaches and deeper detection implantation, they typically achieve higher signal fidelity at the cost of increased surgical risk, ethical considerations, and implementation complexity.
Company Approaches Within the Framework
Applying this framework to the three subject companies reveals their distinct technological positioning:
Paradromics occupies the Invasive/Implantation quadrant, prioritizing signal quality through direct neural recording. This approach requires experienced neurosurgeons and carries higher surgical risk but offers the theoretical maximum for signal fidelity [90] [4].
Precision Neuroscience positions in the Minimally invasive/Implantation space, balancing respectable signal quality with reduced surgical trauma. Their cortical surface approach provides higher channel count than vascular approaches while avoiding parenchymal penetration [93] [94].
Synchron operates in the Minimally invasive/Intervention category, utilizing the body's natural vasculature as an access route to the brain. This approach offers significantly lower surgical risk while maintaining reasonable signal quality through proximity to neural tissue [95] [89].
Experimental Protocols and Methodologies
Paradromics Connect-One Early Feasibility Study
Study Objectives and Design: The Connect-One Study is designed as an early feasibility study to evaluate the long-term use of the Connexus BCI for restoring speech and enabling computer control in people with severe motor impairments. The study will assess safety, performance, and reliability parameters with initial focus on two participants who have impaired speech and limited extremity movement due to severe loss of voluntary motor control [90] [91].
Participant Selection Criteria: Eligible participants must have speech impairment and limited upper/lower extremity movement due to conditions such as spinal cord injuries, stroke, or ALS. Practical considerations require participants to live within four hours of clinical sites (UC Davis, Massachusetts General Hospital, or University of Michigan) to ensure ongoing monitoring and support [90].
Technical Methodology: The experimental protocol involves surgical implantation of the Connexus BCI system, which includes micro-electrodes placed for neural signal recording and a compact receiver implanted in the chest. During acute implantation studies conducted previously at the University of Michigan, the team demonstrated that the Connexus BCI could be safely implanted, record brain signals, and be removed intact in less than 20 minutes—a significant technical milestone [91].
Data Collection and Analysis: The system records neural signals at unprecedented rates of information transfer, with preclinical models demonstrating 200+ bits per second. Recorded signals are processed by external artificial intelligence systems that translate brain activity into intended speech or computer commands. The study will collect both quantitative data (information transfer rates, accuracy metrics) and qualitative clinical outcomes related to communication restoration [90].
Precision Neuroscience FDA Clearance Studies
Study Objectives and Design: Precision's studies leading to FDA clearance focused on demonstrating the safety and efficacy of the Layer 7 Cortical Interface for individuals with paralysis. The non-penetrating design and minimal tissue disruption were key safety considerations, while efficacy was evaluated through device control tasks [93] [94].
Technical Methodology: The Layer 7 array's 1,024 electrodes embedded in a flexible film provide high-channel-count recording from the cortical surface. The array conforms to the brain surface without penetrating tissue, reducing immune response and potential damage. The FDA clearance specified implantation durations of up to 30 days, indicating the focus on temporary rather than permanent implantation [93].
Data Collection and Analysis: Signal quality and stability were assessed through motor intent decoding tasks, where participants attempted to control digital devices through thought. The high electrode density enables spatially precise mapping of cortical activity, potentially allowing for more nuanced decoding of movement intentions compared to lower-channel-count systems [93].
Synchron Clinical Trials
Study Objectives and Design: Synchron's clinical trials have enrolled 10 patients with paralysis across sites in the U.S. and Australia. The studies focus on individuals with severe paralysis who retain intact cognitive function and can generate motor-intent signals, particularly targeting conditions like ALS and other upper-motor-neuron diseases where intent is preserved [95].
Participant Selection Criteria: Candidates include individuals with paralysis from spinal cord injury or stroke, provided their motor cortex remains structurally and functionally intact after injury. Suitability depends more on the ability to consistently produce motor-intent signals for decoding than on the specific underlying cause of paralysis [95].
Technical Methodology: The Stentrode device is implanted via endovascular catheterization through the blood vessels, without open-brain surgery. The procedure involves navigating the device through the venous system to position it adjacent to the motor cortex. Once in place, the platform interfaces with the motor cortex through the blood vessel walls, recording neural signals that are wirelessly transmitted for decoding [95].
Data Collection and Analysis: The system translates recorded cortical activity into digital commands for hands-free control of digital devices. Integration with Bluetooth technology enables direct connection to Apple electronics, providing practical communication solutions for paralyzed individuals. Additional clinical trials are planned to expand evidence generation and refine the fully implanted system [95].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Research Reagent Solutions in BCI Development
| Material/Component | Function | Example Implementation |
|---|---|---|
| Platinum-Iridium Electrodes | Neural signal recording with high biocompatibility and conductivity | Paradromics uses these materials in their micro-electrodes for long-term implantation [90] |
| Flexible Polymer Substrates | Thin, conformable backing for electrode arrays enabling cortical surface contact | Precision's Layer 7 interface uses an ultra-thin film (1/5 human hair thickness) [93] |
| Medical-Grade Titanium | Hermetic encapsulation and structural components for implanted devices | Paradromics utilizes titanium in their implantable receiver for biocompatibility [90] |
| Endovascular Stent Material | Nitinol or similar alloy providing scaffold for transvascular electrodes | Synchron's Stentrode uses stent-based platform for vascular access [95] |
| Wireless Transceiver Systems | Transcutaneous data transmission between implanted and external components | Paradromics chest unit wirelessly transmits data through the skin [90] |
| Biocompatible Encapsulants | Protective coatings preventing tissue damage and immune response | All three companies utilize specialized materials for long-term biocompatibility [90] [93] [95] |
| AI-Decoding Algorithms | Signal processing and translation of neural activity to digital commands | Synchron is growing an AI team to train models for real-time thought decoding [89] |
The BCI landscape is characterized by diverse approaches to solving the fundamental challenge of balancing signal fidelity with procedural safety. Paradromics, Precision Neuroscience, and Synchron represent distinct points on this spectrum, each with technical trade-offs that make them suitable for different clinical applications and patient populations.
Future development in the field will likely focus on several key areas: (1) increasing channel counts and information transfer rates while minimizing tissue disruption; (2) improving biocompatibility for long-term implantation stability; (3) enhancing artificial intelligence capabilities for more natural and intuitive neural decoding; and (4) developing closed-loop systems that can both record from and stimulate neural tissue for bidirectional communication [4] [96] [89].
The significant investments in these companies—including Synchron's $200 million funding round, Paradromics' partnership with NEOM, and Precision Neuroscience's backing from SCI Ventures—demonstrate strong confidence in the clinical and commercial potential of BCI technologies [93] [89] [92]. As these technologies mature, they hold promise not only for restoring function in neurological disorders but potentially for enhancing human capabilities through direct neural interaction with intelligent systems, immersive digital environments, and advanced prosthetics [90].
Brain-Computer Interfaces (BCIs) represent a revolutionary technology that establishes a direct communication pathway between the brain and external devices [54]. For individuals with severe neurological disorders, BCIs offer the potential to restore lost functions, enabling control of assistive devices and communication through direct neural signal decoding [9]. The transition of BCI technology from laboratory research to validated clinical intervention requires rigorous evaluation through well-designed human studies that systematically assess both safety and efficacy endpoints [1].
The clinical development of BCIs occurs within an evolving regulatory framework that is adapting to accommodate neurotechnological innovations. Global health authorities have implemented regulatory updates to streamline clinical trial processes, with recent developments including the adoption of ICH E6(R3) Good Clinical Practice guidelines that introduce more flexible, risk-based approaches to trial design and conduct [97]. China's National Medical Products Administration (NMPA) has implemented revisions to clinical trial regulations aimed at accelerating drug development and shortening approval timelines by approximately 30%, while also allowing for adaptive trial designs with real-time protocol modifications under appropriate safety oversight [97]. These regulatory advancements create an environment conducive to evaluating innovative BCI technologies while maintaining rigorous patient protection standards.
BCI Working Principles and Signal Acquisition Foundations
Core Components of BCI Systems
At its fundamental level, a brain-computer interface is a system that measures central nervous system activity and converts it into artificial outputs that replace, restore, enhance, supplements, or improves natural CNS outputs [98]. All BCI systems share a common operational pipeline consisting of four critical components: (1) signal acquisition, (2) signal processing and decoding, (3) output device control, and (4) feedback mechanisms that create a closed-loop system [1].
The signal acquisition phase involves capturing neural signals through various interface technologies. Signal processing algorithms then filter noise and extract relevant features from the raw neural data. Decoding algorithms translate these processed signals into interpretable commands that reflect user intent. These commands subsequently control external devices, such as computer cursors, communication interfaces, or prosthetic limbs. Finally, the system provides sensory feedback to the user, enabling adjustment of mental strategies to improve control [1].
Signal Acquisition Methodologies
BCI signal acquisition methods are categorized based on their degree of invasiveness and spatial resolution:
- Non-invasive BCIs: Utilize electrodes placed on the scalp surface to record electroencephalography (EEG) signals. This approach is safe and convenient but suffers from limited spatial resolution due to signal attenuation through intervening tissues [12].
- Invasive BCIs: Involve microelectrode arrays implanted directly into brain tissue, enabling recording of single-neuron activity with high spatial and temporal resolution [1]. Examples include the Utah array and Neuralink's implantable chip [1].
- Semi-invasive BCIs: Occupy an intermediate position, with electrodes placed inside the skull but not penetrating brain tissue. Examples include electrocorticography (ECoG) grids and endovascular approaches like Synchron's Stentrode, which is delivered via blood vessels [1] [12].
Table 1: Comparison of BCI Signal Acquisition Technologies
| Acquisition Method | Spatial Resolution | Temporal Resolution | Key Advantages | Primary Limitations |
|---|---|---|---|---|
| Non-invasive (EEG) | Low (~1-2 cm) | High (ms) | Safe, portable, low cost | Low signal quality, susceptibility to artifacts |
| Invasive (Microelectrodes) | High (μm) | High (ms) | High-quality signals, single-neuron recording | Surgical risk, tissue response, signal stability |
| Semi-invasive (ECoG) | Moderate (mm) | High (ms) | Better signal quality than EEG, reduced risk compared to invasive | Still requires surgery, lower resolution than invasive |
| Endovascular (Stentrode) | Moderate (mm) | High (ms) | No open brain surgery, signals from cortical surface | Limited to vessels near target areas, long-term patency concerns |
The core technological challenge in BCI signal acquisition lies in maximizing information bandwidth while minimizing biological risk. Recent advances include flexible neural interfaces that reduce mechanical mismatch with brain tissue, high-channel-count recording systems that capture more neural information, and wireless transmission technologies that enhance user mobility [54] [12].
Clinical Trial Design for BCI Systems
Efficacy Endpoints in BCI Trials
Demonstrating meaningful clinical benefit represents the primary objective of BCI trials. Efficacy assessment incorporates both performance-based metrics and clinically validated functional outcome measures:
- Upper Limb Motor Function: For motor restoration applications, the Fugl-Meyer Assessment (FMA) for upper extremities serves as a gold standard outcome measure, particularly in stroke rehabilitation trials [99]. The Action Research Arm Test (ARAT) provides additional assessment of functional hand and arm movements.
- Activities of Daily Living: The Modified Barthel Index (MBI) evaluates independence in self-care, mobility, and other daily activities, providing insight into real-world functional impact [99].
- Communication Performance: For communication BCIs, metrics include characters per minute, selection accuracy, and information transfer rate, which quantify practical communication capacity [1].
- Neurophysiological Outcomes: Measures such as functional connectivity changes on fMRI or EEG coherence provide insights into neural mechanisms underlying functional improvements [99].
Recent evidence from systematic reviews indicates that BCI-combined therapy can significantly improve upper limb motor function and quality of daily life for stroke patients, demonstrating particularly promising results in the subacute phase of recovery [99].
Safety Monitoring and Adverse Event Characterization
Comprehensive safety assessment in BCI trials encompasses both procedure-related and device-related risks:
- Procedure-Related Risks: For invasive BCIs, these include surgical complications (hemorrhage, infection), postoperative sequelae (pain, swelling), and anesthesia-related adverse events [1].
- Device-Related Risks: Encompass biological responses to implanted materials (inflammation, glial scarring, foreign body reaction), device failure (electrode malfunction, connection integrity), and long-term stability concerns [1].
- Operation-Related Risks: Include unintended device behaviors, cybersecurity vulnerabilities, and potential neurological effects of prolonged stimulation or recording [9].
Safety monitoring extends throughout the trial duration and includes systematic assessment of serious adverse events, device deficiencies, and unanticipated adverse device effects. Recent trials of minimally invasive approaches, such as Synchron's Stentrode, have reported favorable safety profiles with no serious adverse events or blood vessel blockages observed at 12-month follow-up in a four-patient trial [1].
Innovative Trial Designs for Small Populations
BCI trials frequently target patient populations with specific neurological conditions, resulting in limited participant availability. This constraint has prompted adoption of innovative trial designs:
- Adaptive Designs: Permit modifications to trial parameters based on interim results without compromising validity, enabling more efficient evaluation in small cohorts [97].
- Bayesian Approaches: Incorporate prior knowledge and continuously update probability assessments as data accumulates, potentially reducing required sample sizes [97].
- Single-Subject Designs: Employ rigorous N-of-1 methodologies with extensive baseline assessment and multiple crossover phases to demonstrate individual efficacy [1].
Regulatory agencies have recognized the need for flexible approaches to trial design for small populations. The U.S. FDA has issued draft guidance recommending novel trial designs and endpoints to support product licensure for rare conditions, encouraging use of innovative statistical approaches and surrogate endpoints to efficiently generate evidence [97].
Quantitative Assessment of BCI Clinical Evidence
Systematic Review of Stroke Rehabilitation Trials
A 2025 overview of systematic reviews evaluated the methodological quality and evidence supporting BCI interventions for stroke rehabilitation [99]. After screening 908 initially identified articles, 18 studies met inclusion criteria for qualitative and quantitative synthesis. The re-evaluation using AMSTAR-2 and PRISMA criteria determined that the quality of systematic reviews and meta-analyses concerning stroke BCI training is moderate, providing relatively good evidence for clinical decision-making [99].
Table 2: Efficacy Outcomes from BCI Stroke Rehabilitation Trials
| Functional Domain | Assessment Scale | Evidence Level | Clinical Significance | Population with Greatest Benefit |
|---|---|---|---|---|
| Upper Limb Motor Function | Fugl-Meyer Assessment (FMA) | Strong | Statistically significant improvements | Subacute stroke, severe impairment |
| Activities of Daily Living | Modified Barthel Index (MBI) | Moderate | Meaningful functional improvements | Subacute and chronic stroke |
| Lower Limb Motor Function | Various mobility scales | Limited | Inconsistent evidence | Requires further study |
| Speech Function | Speech articulation metrics | Limited | Insufficient evidence | Requires further study |
| Long-Term Outcomes | Retention assessments | Limited | Insufficient follow-up data | Multicenter, long-term studies needed |
The analysis concluded that BCI-combined treatment demonstrates good safety and can improve upper limb motor function and quality of daily life for stroke patients, especially those in the subacute phase [99]. However, effects on improving speech function, lower limb motor function, and long-term outcomes require further evidence from larger, more rigorous trials.
Current Clinical Trial Landscape
As of mid-2025, the BCI field is experiencing rapid clinical translation, with approximately 90 active human trials testing implants for various applications including communication, mobility, and stroke rehabilitation [1]. These trials span North America, Europe, Asia, and Australia, involving an unprecedented number of human participants [1]. Key players advancing to clinical stages include:
- Neuralink: Received FDA clearance in 2023 to begin human trials, with five individuals with severe paralysis reportedly using the system to control digital and physical devices by June 2025 [1].
- Synchron: Conducted a four-patient trial of its Stentrode endovascular BCI, demonstrating the ability for participants with paralysis to control computers for texting and other functions [1].
- Precision Neuroscience: Received FDA 510(k) clearance in April 2025 for its Layer 7 cortical interface, authorized for commercial use with implantation durations of up to 30 days [1].
- Paradromics: Partnered with the University of Michigan to perform first-in-human recording with its Connexus BCI device in a patient undergoing epilepsy surgery, with plans to launch a full clinical trial by late 2025 [1].
No BCI system has yet received general medical use approval, with all current devices remaining in experimental stages [1]. The field's current position resembles that of gene therapies in the 2010s or heart stents in the 1980s—on the cusp of transitioning from experimental status to regulated clinical use [1].
Experimental Protocols and Methodologies
Standardized BCI Rehabilitation Protocol
For clinical trials investigating BCI for motor recovery after stroke, a typical experimental protocol incorporates the following key elements:
- Participant Selection: Recruitment of patients with confirmed stroke diagnosis, stratified by time since onset (acute, subacute, or chronic) and severity of impairment [99]. Inclusion criteria typically specify focal motor deficits in upper extremities with preserved cognitive capacity to engage with the BCI system.
- Baseline Assessment: Comprehensive evaluation using standardized scales (FMA, ARAT, MBI) conducted by blinded assessors, complemented by neurophysiological assessment (EEG, fMRI) to characterize baseline brain state and connectivity [99].
- Intervention Protocol: BCI training sessions typically conducted 3-5 times weekly for 4-12 weeks, with each session lasting 60-90 minutes [99]. The protocol combines motor imagery with real-time feedback provided through visual or tactile modalities. BCI systems are often integrated with functional electrical stimulation or robotic devices to provide contingent movement assistance when intended motor imagery is detected.
- Control Condition: Comparison with dose-matched conventional therapy or sham feedback conditions to isolate specific BCI effects from general therapeutic benefits [99].
- Outcome Assessment: Repeated evaluation at predetermined intervals (immediately post-intervention, 3-month, and 6-month follow-up) using the same standardized measures employed at baseline [99].
Signal Processing and Decoding Workflow
The transformation of raw neural signals into device commands follows a standardized processing pipeline:
Diagram: BCI Signal Processing and Control Pipeline
The decoding algorithms typically employ machine learning approaches, with deep learning methods increasingly applied to achieve higher accuracy in complex decoding tasks such as speech reconstruction [1]. Recent advances have demonstrated speech BCIs capable of inferring words from complex brain activity at 99% accuracy with less than 0.25 second latency—performance levels that were unattainable just a decade earlier [1].
Research Reagent Solutions for BCI Development
Table 3: Essential Research Materials and Technologies for BCI Development
| Research Tool Category | Specific Examples | Primary Function | Application in BCI Development |
|---|---|---|---|
| Neural Signal Acquisition Chips | SX-R128S4 high-throughput neural signal acquisition chip [12] | High-sensitivity recording of weak neural signals | Captures brain neural signals at microvolt level with excellent noise suppression |
| Signal Processing Units | Custom ASICs or FPGA-based processors [12] | Real-time signal processing and feature extraction | Performs amplification, filtering, and analog-to-digital conversion of neural signals |
| Neural Electrodes | Utah array, Neuralace, micro-electrode arrays [1] | Interface with neural tissue for signal recording | Provides high-density neural recording from cortical surfaces or within brain tissue |
| Wireless Transmission Systems | SX-WD60 low-power wireless transmission chip [12] | Data communication without physical tethering | Enables transmission of processed brain signals to external devices |
| Decoding Algorithms | Deep learning architectures (CNNs, RNNs) [1] | Translation of neural signals to intended commands | Interprets user intent from brainwave patterns for device control |
| Biocompatible Materials | Flexible polymer substrates, hydrogel coatings [54] | Interface between device and neural tissue | Enhances long-term biocompatibility and reduces immune response |
Regulatory and Ethical Considerations
Evolving Regulatory Frameworks
BCI technologies navigate a complex regulatory landscape that varies across jurisdictions. Recent developments include:
- United States FDA: Has established expedited programs for regenerative medicine therapies, which may provide relevant precedents for advanced BCI technologies [97]. The agency has also issued draft guidance on innovative trial designs for small populations, recognizing the need for flexible approaches when evaluating therapies for rare conditions [97].
- European Medicines Agency: Has emphasized the importance of including patient experience data throughout the medical product lifecycle, from pre-authorization through post-authorization benefit-risk evaluation [97].
- China NMPA: Has implemented significant regulatory reforms to accelerate innovative device approval, including revised clinical trial policies that streamline development and shorten approval timelines [97].
The International Council for Harmonisation (ICH) has advanced global harmonization through updated guidelines including ICH E6(R3) for Good Clinical Practice and ICH E2D(R1) for post-approval safety data management [97].
Ethical Implementation in Clinical Trials
Ethical conduct of BCI trials requires attention to several unique considerations:
- Informed Consent: Ensuring genuine understanding of risks and potential benefits, particularly when recruiting participants with communication impairments [54]. Processes must be adapted to accommodate specific disabilities while maintaining rigorous consent standards.
- Privacy and Data Security: Protecting exceptionally sensitive neural data requires robust cybersecurity measures and clear policies regarding data ownership and usage [9].
- Risk-Benefit Assessment: Balancing potential functional restoration against invasive procedure risks, particularly for devices with limited long-term safety data [54].
- Post-Trial Access: Addressing obligations to continue providing access to beneficial interventions after trial conclusion, particularly for participants who become dependent on the technology [54].
Emerging Trends in BCI Clinical Development
The clinical trial landscape for BCIs is evolving rapidly, with several notable trends shaping future development:
- Hybrid Approaches: Integration of BCIs with other emerging technologies, including virtual reality for enhanced neurorehabilitation and artificial intelligence for improved decoding algorithms [54].
- Personalized Digital Prescription Systems: Development of customized therapeutic strategies delivered via digital platforms, enabling individually optimized intervention parameters [54].
- Closed-Loop Neurostimulation: Systems that not only record neural signals but also provide targeted stimulation based on detected brain states, creating bidirectional brain-computer interfaces [54].
- Global Market Expansion: Increasing international participation in BCI development, with particularly rapid growth in China's BCI initiatives supported by recent policy implementations [12] [100].
The clinical trial landscape for brain-computer interfaces represents a rapidly advancing frontier where technological innovation, clinical science, and regulatory policy converge. Current evidence supports the efficacy and safety of BCI approaches for specific applications, particularly upper limb motor rehabilitation after stroke [99]. The field is transitioning from proof-of-concept demonstrations to rigorous clinical validation, with multiple companies advancing toward regulatory submissions [1].
Future progress will depend on addressing key challenges, including standardization of outcome measures, demonstration of long-term benefits, and collection of robust safety data across diverse patient populations. As the technology matures, BCIs hold exceptional promise to restore function and improve quality of life for individuals with severe neurological impairments, potentially revolutionizing neurorehabilitation and human-technology interaction.
The efficacy of a Brain-Computer Interface (BCI) system is fundamentally contingent upon the initial stage of neural signal acquisition. This process entails the detection and recording of electrophysiological or hemodynamic activity from the brain, which is subsequently translated into commands for external devices [8] [6]. The working principle of a BCI rests on establishing a direct communication pathway between the human brain and an external device, bypassing conventional neuromuscular outputs [8]. This pathway can be categorized into invasive, semi-invasive, and non-invasive systems, each with distinct trade-offs between signal fidelity, spatial resolution, and clinical risk [8] [86]. The core challenge in BCI signal acquisition lies in balancing these factors while advancing towards higher channel counts, better biocompatibility, and lower power consumption—areas where material innovations, hybrid systems, and low-power chip design are proving pivotal.
Material Innovations for Next-Generation Neural Interfaces
Material science is driving progress in neural interfaces by addressing critical issues of biocompatibility, signal stability, and chronic reliability. The evolution of electrode materials and encapsulation substrates is enabling more intimate and stable interfaces with neural tissue.
Advanced Electrode Materials and Encapsulation
Innovations in electrode design focus on maximizing signal quality while minimizing the immune response. Flexible polymer-based substrates and nanomaterial electrodes are being developed to reduce the mechanical mismatch between rigid implants and soft brain tissue, thereby mitigating chronic immune response and glial scar formation [101]. A key innovation involves the use of stacked silicon–polymer hybrid probes. Chinese research teams, for instance, are creating probes that stack thin-film polymer electrode layers on a silicon carrier wafer, achieving 2,048 independent recording sites in a compact 4 × 4 mm footprint—a near fourfold density improvement over some contemporary designs [101].
For long-term implantation, hermetic encapsulation is paramount. State-of-the-art solutions employ alternating atomic-layer deposition (ALD) stacks of aluminum oxide (Al₂O₃) and hafnium oxide (HfO₂), with a total thickness of approximately 2 µm. Accelerated aging tests in saline baths at 60°C predict less than 1% moisture ingress after a decade, a level of protection on par with cardiac pacemakers [101]. For non-invasive systems, the development of dry electrodes using materials like carbon nanotubes and conductive polymers is overcoming the traditional need for conductive gels, improving user convenience and enabling integration into wearable form factors such as headbands and AR/VR headsets [86].
Research Reagent Solutions for Neural Interface Development
Table 1: Key Materials and Reagents for Advanced BCI Development
| Material/Reagent | Primary Function | Application in BCI R&D |
|---|---|---|
| Liquid Crystal Polymer (LCP) | Flexible interconnect substrate | Used in implantable arrays for its neural tissue-like Young's modulus (~2 GPa), reducing micromotion-induced gliosis [101]. |
| ALD Al₂O₃/HfO₂ Stack | Hermetic encapsulation barrier | Protects implantable chips from moisture and ions; target is <1% moisture ingress over 10-year device lifetime [101]. |
| Conductive Hydrogels | Biocompatible electrode coating | Improves signal transduction and reduces impedance at the tissue-electrode interface for chronic implants [8]. |
| Carbon Nanotube-based Inks | Dry electrode conductor | Enables comfortable, gel-free, high-quality EEG recording for non-invasive consumer and medical wearables [86]. |
Hybrid BCI Systems and Multi-Modal Signal Acquisition
Hybrid BCIs integrate multiple neural signal acquisition technologies or combine BCIs with other physiological interfaces to enhance system reliability, information transfer rate, and overall usability.
Architectures and Signal Fusion Methodologies
The core principle of a hybrid BCI is to leverage the complementary strengths of different signal types. A common architecture merges Electroencephalography (EEG), which offers high temporal resolution, with functional Near-Infrared Spectroscopy (fNIRS), which provides better spatial resolution and measures hemodynamic activity [8] [86]. This fusion can compensate for the limitations of each modality when used independently. Other hybrid configurations integrate BCIs with eye-tracking, electromyography (EMG), or electrooculography (EOG) to create more robust assistive technologies [8] [102].
The experimental workflow for developing and validating a hybrid BCI typically follows a structured, iterative process, as outlined below.
Diagram 1: Hybrid BCI experimental workflow.
A detailed methodology for a hybrid EEG-fNIRS experiment, as referenced in the workflow, would involve:
Step 1: Multi-Modal Signal Acquisition
- Equipment Setup: Simultaneously deploy a high-density EEG cap (e.g., 64+ channels) and a fNIRS headset with overlapping optode placement. Ensure synchronization of data streams using a hardware trigger or software timestamp.
- Experimental Paradigm: Design a task that elicits robust, spatially localized brain activity, such as a motor imagery (e.g., imagining hand movement) or a cognitive task.
Step 2: Signal Pre-processing
- EEG Pipeline: Apply a band-pass filter (e.g., 0.5-40 Hz), notch filter (50/60 Hz), and artifact removal algorithms (e.g., Independent Component Analysis (ICA) to eliminate ocular and muscle artifacts).
- fNIRS Pipeline: Convert raw light intensity signals to optical density, then to concentration changes of oxygenated (HbO) and deoxygenated hemoglobin (HbR) using the Modified Beer-Lambert Law.
Step 3: Feature Extraction
- EEG Features: Extract power spectral density in specific frequency bands (e.g., Mu rhythm: 8-12 Hz, Beta rhythm: 13-30 Hz) from relevant electrodes.
- fNIRS Features: Calculate the mean, slope, and variance of the HbO and HbR time-series during task periods.
Step 4: Data Fusion & Decoding
- Fuse the extracted EEG and fNIRS feature vectors into a single, high-dimensional dataset.
- Train a machine learning classifier (e.g., Support Vector Machine (SVM) or Linear Discriminant Analysis (LDA)) on this fused dataset to decode the user's intent.
Step 5 & 6: Application and Validation
- The classifier's output is translated into a command for an external device (e.g., a prosthetic limb or a computer cursor).
- System performance is rigorously quantified using metrics like classification accuracy, information transfer rate (ITR), and false positive rate, leading to iterative refinement.
Low-Power Chip Design for Implantable and Wearable BCIs
The development of ultra-low-power, high-density chips is critical for the viability of implantable BCIs, which require years of operation without bulky external power sources, and for wearable devices that demand user comfort and long battery life.
Architectural Innovations for Power Efficiency
The power budget for implantable systems is exceptionally constrained, often targeting under 5 mW per channel for devices with thousands of channels [101]. To achieve this, system-on-chip (SoC) designs integrate several key innovations:
- On-Chip Analog Front-End (AFE): The signal chain begins with an ultra-low-noise amplifier (ULNA). Designs employing chopper-stabilization achieve input-referred noise of less than 2 µVrms, which is essential for capturing microvolt-scale neural signals, while maintaining high dynamic range (>80 dB) and integrated DC-offset cancellation [101].
- Near-Sensor Computing: To drastically reduce data transmission power—which typically dominates total power consumption—advanced chips perform feature extraction and compression directly on the chip. Techniques include event-based thresholding, where only data samples crossing an adaptive threshold are packetized, reducing throughput by ~70%, and the implementation of lightweight Principal Component Analysis (PCA) cores to compress multi-unit activity before transmission [101].
- Wireless Telemetry and Power Management: Implantable systems often use a dual-band approach: Ultra-Wideband (UWB) in the 6–8 GHz range for high-speed neural data (up to 2 Gbps raw throughput), and Inductive Power Transfer (IPT) at 13.56 MHz to wirelessly deliver up to 500 mW to the implant. Custom-designed power management ICs (PMICs) with synchronous rectifiers achieve end-to-end efficiencies as high as 90% [101].
National Roadmaps and Quantitative Performance Targets
China's state-backed BCI initiative explicitly targets the development of core chips with high channel counts and low power consumption [102] [103]. Companies like NeuraMatrix have already developed 128-channel SoCs for implantable applications [102]. The broader global market reflects this push for miniaturization and efficiency, with the overall BCI market projected to grow from USD 3.2 billion in 2024 in China to USD 5.58 billion by 2027, a compound annual growth rate (CAGR) of 20% [102]. IDTechEx further forecasts the global BCI market to surpass USD 1.6 billion by 2045, with a CAGR of 8.4% from 2025 [86].
Table 2: Quantitative Benchmarks for Next-Generation BCI Chips (Invasive)
| Performance Parameter | Current State-of-the-Art (2025) | Forward-looking Targets (2027-2030) | Primary Challenge |
|---|---|---|---|
| Channel Count | 1,024 - 3,000 channels [101] | > 10,000 channels [101] | Wiring density, heat dissipation, data bandwidth. |
| Power Consumption | < 5 mW per channel [101] | Sub-1 mW per channel | Maintaining signal integrity while scaling down power. |
| Input-Referred Noise | < 2 µVrms [101] | < 1.5 µVrms | Minimizing noise in ultra-low-power amplifier designs. |
| On-Chip Data Compression | ~70% reduction via event-based sampling [101] | >85% lossless compression | Developing low-complexity, high-efficiency algorithms. |
| Wireless Data Rate | Up to 2 Gbps (UWB) [101] | 5-10 Gbps | Overcoming tissue attenuation at higher frequencies. |
The relationship between these key technological pillars and their collective impact on BCI performance and application can be visualized as an interconnected system.
Diagram 2: Interdependence of core technological pillars in BCI development.
The future trajectory of brain-computer interfaces is being fundamentally shaped by concurrent advances in material innovations, hybrid systems, and low-power chip design. These pillars are not developing in isolation but are deeply interdependent, collectively pushing the boundaries of what is possible in human-machine integration. Materials science provides the foundation for safe and effective long-term interfacing, hybrid system architectures offer a path to greater robustness and utility, and ultra-low-power chip technology is the key to unlocking portable, high-channel-count, and fully implantable systems. As these roadmaps converge, they promise to transform BCI technology from a primarily assistive tool into a platform with broad applications in healthcare, communication, and human augmentation. The ongoing, policy-driven efforts on a global scale, exemplified by China's ambitious 2027 and 2030 targets, underscore the strategic importance of these technological domains and signal an accelerated pace of innovation and commercialization in the coming years [102] [103].
Conclusion
Brain-Computer Interface technology has matured from a laboratory concept to a field with significant clinical and commercial momentum. The foundational principles of translating neural activity into device commands are now being realized through diverse signal acquisition methods, each presenting a distinct balance of signal quality, invasiveness, and implementation complexity. While challenges in signal stability, biocompatibility, and data processing persist, advancements in materials science, AI-driven decoding, and minimally invasive surgical techniques are paving the way for more robust and accessible systems. For biomedical researchers and clinicians, this evolution promises not only powerful new tools for restoring function in patients with neurological disorders but also novel platforms for understanding brain function and accelerating therapeutic development. The future of BCI lies in the continued convergence of engineering and neuroscience, leading to more personalized, high-fidelity, and seamlessly integrated human-machine systems.
References
- 1. BCIs in 2025: Trials, Progress, and Challenges [https://andersenlab.com/blueprint/bci-challenges-and-opportunities]
- 2. Brain–computer interface: trend, challenges, and threats - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC10403483/]
- 3. Challenges and Opportunities for the Future of Brain ... [https://www.frontiersin.org/journals/neuroscience/articles/10.3389/fnins.2021.699428/full]
- 4. Signal acquisition of brain–computer interfaces - PMC - NIH [https://pmc.ncbi.nlm.nih.gov/articles/PMC11955058/]
- 5. A Comprehensive Survey of Brain–Computer Interface ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12180781/]
- 6. Review Signal acquisition of brain–computer interfaces [https://www.sciencedirect.com/science/article/pii/S2667325824001559]
- 7. Signal Acquisition Methods and Interaction Paradigms [https://www.arxiv.org/abs/2503.16471]
- 8. The Global Brain-Computer Interfaces Market 2025-2035 [https://www.futuremarketsinc.com/the-global-brain-computer-interfaces-market-2025-2035/]
- 9. Global Brain-Computer Interfaces Markets 2025-2035 [https://www.businesswire.com/news/home/20240910576546/en/Global-Brain-Computer-Interfaces-Markets-2025-2035-Signal-Acquisition-Methods-and-Neural-Interfaces---Revolutionizing-Neuroprosthetics-and-Rehabilitation---ResearchAndMarkets.com]
- 10. The Future of Brain-Computer Interfaces: What to Expect [https://boomset.com/the-future-of-brain-computer-interfaces-what-to-expect/]
- 11. Advancing Brain-Computer Interface Closed-Loop Systems for ... [https://biomedeng.jmir.org/2025/1/e72218]
- 12. Brain-Computer Chip: Ushering in the First Year of ... [https://eu.36kr.com/en/p/3423925429816962]
- 13. Secure wireless communication of brain–computer ... [https://www.nature.com/articles/s41467-025-63326-0]
- 14. A High-DOF BCI Control Strategy Mapping Discrete ... [https://ui.adsabs.harvard.edu/abs/2025ITASE..2220234M/abstract]
- 15. Brain-computer interfaces are closer than you think [https://www.clinicaltrialsarena.com/analyst-comment/brain-computer-interfaces-closer/]
- 16. A brain-computer interface roadmap for diagnosing and ... [https://www.the-innovation.org/article/doi/10.59717/j.xinn-inform.2025.100016]
- 17. Thinking about the action potential: the nerve signal as a ... [https://www.frontiersin.org/journals/cellular-neuroscience/articles/10.3389/fncel.2023.1232020/full]
- 18. The origin of extracellular fields and currents — EEG, ECoG ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4907333/]
- 19. Possible mechanism of action potential propagation ... [https://www.sciencedirect.com/science/article/pii/S2405844024136687]
- 20. A quantitative model reveals a frequency ordering of ... [https://www.nature.com/articles/s42003-022-04049-6]
- 21. Brain-computer interface paradigms and neural coding - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC10822902/]
- 22. Brain–computer interface [https://en.wikipedia.org/wiki/Brain%E2%80%93computer_interface]
- 23. A Review of Brain-Computer Interface Technologies [https://arxiv.org/html/2503.16471v1]
- 24. Motor imagery-based brain–computer interface rehabilitation ... [https://jneuroengrehab.biomedcentral.com/articles/10.1186/s12984-024-01387-w]
- 25. Improving Motor Imagery-Based Brain-Computer Interface ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8602688/]
- 26. Motor task-to-task transfer learning for motor imagery brain- ... [https://www.sciencedirect.com/science/article/pii/S1053811924004038]
- 27. Multi-frequency steady-state visual evoked potential dataset [https://www.nature.com/articles/s41597-023-02841-5]
- 28. The P300-Based Brain-Computer Interface (BCI): Effects of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3050994/]
- 29. Steady state visually evoked potential [https://en.wikipedia.org/wiki/Steady_state_visually_evoked_potential]
- 30. On closed-loop brain stimulation systems for improving the ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10076878/]
- 31. Comprehensive evaluation methods for translating BCI into ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11188342/]
- 32. The history, current state and future possibilities of the non- ... [https://www.sciencedirect.com/science/article/pii/S2590093525000049]
- 33. Invasive vs. Non-Invasive Neuronal Signals for Brain-Machine ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4921501/]
- 34. A New Path to Noninvasive Brain-Computer Interface [https://www.jhuapl.edu/news/news-releases/241114-noninvasive-brain-computer-interface]
- 35. Non-invasive brain-machine interface control with artificial ... [https://pubmed.ncbi.nlm.nih.gov/39416032/]
- 36. AI Co-Pilot Boosts Noninvasive Brain-Computer Interface ... [https://samueli.ucla.edu/ai-co-pilot-boosts-noninvasive-brain-computer-interface-by-interpreting-user-intent/]
- 37. Exploring Imagined Movement for Brain–Computer Interface ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12468679/]
- 38. fNIRS vs EEG: Comparing Non-Invasive Brain Imaging ... [https://imotions.com/blog/learning/research-fundamentals/fnirs-vs-eeg/?srsltid=AfmBOooRJ9fBEQXetlRol2MOsxz_GJFlkVJi2pY0N-CiQczH8o29y2mF]
- 39. EEG–fNIRS signal integration for motor imagery ... [https://www.sciencedirect.com/science/article/pii/S2772528625000299]
- 40. The applications of BCI in the Treatment of Mental ... [https://www.deanfrancispress.com/index.php/te/article/view/2564]
- 41. Personalized Brain–Computer Interface and Its Applications [https://pmc.ncbi.nlm.nih.gov/articles/PMC9861730/]
- 42. Artificial Intelligence Approaches for EEG Signal Acquisition ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12390113/]
- 43. The Stentrode™ System by Synchron: Architectural Design ... [https://www.auctoresonline.org/article/the-stentrode-system-by-synchron-architectural-design-and-clinical-translation-of-a-minimally-invasive-braincomputer-interface]
- 44. Advances in endovascular brain computer interface [https://www.sciencedirect.com/science/article/pii/S0165027025001128]
- 45. Advances in large-scale electrophysiology with high-density ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12394932/]
- 46. Advances in flexible high-density microelectrode arrays for ... [https://www.sciencedirect.com/science/article/pii/S0956566325009790]
- 47. High-resolution brain–computer interface with electrode ... [https://www.nature.com/articles/s41551-025-01502-9]
- 48. Optimizing Computer–Brain Interface Parameters for Non- ... [https://www.frontiersin.org/journals/neuroinformatics/articles/10.3389/fninf.2020.00001/full]
- 49. Brain–Machine Interface Projects [https://brain.ieee.org/brain-topics/brain-machine-interface-projects/]
- 50. Design, Fabrication, and Implantation of Invasive ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11340637/]
- 51. Minimally invasive implantation of scalable high-density ... [https://www.nature.com/articles/s41551-025-01501-w]
- 52. DataHigh: Graphical user interface for visualizing and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3950756/]
- 53. Data analysis and visualisation - Modelling, simulation & ... [https://www.ebrains.eu/modelling-simulation-and-computing/visualisation/analysis-and-visualisation/]
- 54. Application and future directions of brain-computer ... [https://www.sciencedirect.com/science/article/pii/S2589238X2500083X]
- 55. Brain-Computer Interfaces in Rehabilitation [https://pubmed.ncbi.nlm.nih.gov/40881516/]
- 56. FDA approves Paradromics' brain-computer interface trial for ... [https://www.statnews.com/2025/11/20/fda-approves-paradromics-bci-trial-for-speech-restoration/]
- 57. Study of promising speech-enabling interface offers hope for ... [https://med.stanford.edu/news/all-news/2025/08/brain-computer-interface.html]
- 58. Quantitative analysis of task selection for brain-computer ... [https://pubmed.ncbi.nlm.nih.gov/25080297/]
- 59. Defining Surgical Terminology and Risk for Brain Computer ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8044752/]
- 60. How Brain-Computer Interfaces Work | BCI Guide [2025] [https://arctop.com/deep-dives/how-bci-works-part-1]
- 61. The functional differentiation of brain–computer interfaces ... [https://www.nature.com/articles/s41599-023-02419-x]
- 62. ECoG, Utah Arrays, and Neuropixels in BCI [https://michaelguth.com/?p=2620]
- 63. Invasive Brain-Computer Interfaces: A Critical Assessment of ... [https://neuro.jmir.org/2024/1/e60151]
- 64. Defining Surgical Terminology and Risk for Brain ... [https://www.frontiersin.org/journals/neuroscience/articles/10.3389/fnins.2021.599549/full]
- 65. Neurosurgical Team Acceptability of Brain–Computer ... [https://www.sciencedirect.com/science/article/pii/S1878875022006921]
- 66. AnEEG: leveraging deep learning for effective artifact ... [https://www.nature.com/articles/s41598-024-75091-z]
- 67. Advancing Brain-Computer Interface Closed-Loop Systems for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12588595/]
- 68. Methods for motion artifact reduction in online brain ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10619676/]
- 69. IMU-Enhanced EEG Motion Artifact Removal with Fine ... [https://arxiv.org/html/2509.01073v1]
- 70. Augmenting brain-computer interfaces with ART: An artifact ... [https://www.sciencedirect.com/science/article/pii/S1053811925001259]
- 71. Toward the integration of mixed reality and brain-computer ... [https://www.sciencedirect.com/science/article/pii/S0208521625000518]
- 72. Revolutionizing brain‒computer interfaces - PubMed Central [https://pmc.ncbi.nlm.nih.gov/articles/PMC12243264/]
- 73. Long-term stability strategies of deep brain flexible neural ... [https://www.nature.com/articles/s41528-025-00410-x]
- 74. The neural tissue around SU-8 implants: A quantitative in ... [https://pubmed.ncbi.nlm.nih.gov/32409039/]
- 75. Study could point way to reducing scar tissue around ... [https://news.uchicago.edu/story/study-could-point-way-reducing-scar-tissue-around-implants]
- 76. Signal Preprocessing, Decomposition and Feature ... [https://www.mdpi.com/2076-3417/15/22/12075]
- 77. Hierarchical attention enhanced deep learning achieves ... [https://www.nature.com/articles/s41598-025-17922-1]
- 78. Long-Term BCI Training of a Tetraplegic User: Adaptive ... [https://www.frontiersin.org/journals/human-neuroscience/articles/10.3389/fnhum.2021.635653/full]
- 79. Online adaptive classification system for brain–computer ... [https://www.sciencedirect.com/science/article/abs/pii/S1746809422010084]
- 80. The Brain-Computer Interface Signals Future Quality of Life ... [https://transmitter.ieee.org/the-brain-computer-interface-signals-future-quality-of-life-improvements/]
- 81. An Online Data Visualization Feedback Protocol for Motor ... [https://pubmed.ncbi.nlm.nih.gov/34163337/]
- 82. Motor imagery based EEG features visualization for BCI ... [https://www.sciencedirect.com/science/article/pii/S1877050918313358]
- 83. BCI Research Documentation Standards for 2025 [https://editverse.com/bci-research-documentation-standards-for-2025/]
- 84. The Past, Present And Future Of Brain-Computer Interfaces [https://www.forbes.com/sites/robtoews/2025/10/05/these-are-the-startups-merging-your-brain-with-ai/]
- 85. The impact of non-invasive brain-computer interface ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12642192/]
- 86. Brain Computer Interfaces 2025-2045: Technologies, ... [https://www.idtechex.com/en/research-report/brain-computer-interfaces/1024]
- 87. What to expect from Neuralink in 2025 [https://www.technologyreview.com/2025/01/16/1110017/what-to-expect-from-neuralink-in-2025/]
- 88. Top 10 Brain-Computer Interface Companies in 2025 [https://www.sphericalinsights.com/blogs/top-10-companies-leading-the-brain-computer-interface-market-in-2025-key-players-statistics-future-trends-2024-2035]
- 89. Synchron raises $200M to prepare for brain computer ... [https://www.medtechdive.com/news/synchron-funding-bci-200m/804977/]
- 90. Paradromics Receives FDA Approval for the Connect-One ... [https://www.paradromics.com/news/paradromics-receives-fda-approval-for-the-connect-one-clinical-study-with-the-connexus-brain-computer-interface]
- 91. Paradromics gets FDA green light for BCI study [https://www.massdevice.com/paradromics-fda-green-light-bci-study/]
- 92. NEOM Investment Fund partners with Paradromics [https://www.neom.com/en-us/newsroom/neom-investment-fund-partners-with-paradromics]
- 93. Precision Neuroscience picks up venture fund backing for ... [https://www.massdevice.com/precision-neursocience-venture-fund-backing-bci/]
- 94. Precision Neuroscience and SCI Ventures Partner to Bring [https://www.globenewswire.com/news-release/2025/11/05/3181536/0/en/Precision-Neuroscience-and-SCI-Ventures-Partner-to-Bring-Brain-Computer-Interfaces-to-People-Living-with-Paralysis.html]
- 95. Synchron raises $200M to advance brain-computer ... [https://www.healio.com/news/neurology/20251114/synchron-raises-200m-to-advance-braincomputer-interface-tech-for-paralysis]
- 96. Global Brain-Computer Interface (BCI) Market Analysis 2025 [https://www.statsandresearch.com/report/40646-global-brain-computer-interface-bci-market]
- 97. Regulatory Updates, September 2025 [https://www.caidya.com/resources/regulatory-updates-sept-2025/]
- 98. Guiding principles and considerations for designing a well ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12119483/]
- 99. Efficacy and safety of brain–computer interface for stroke ... [https://www.frontiersin.org/journals/human-neuroscience/articles/10.3389/fnhum.2025.1525293/full]
- 100. Current landscape of innovative drug development and ... [https://www.nature.com/articles/s41392-025-02267-y]
- 101. China's State-Backed BCI Blueprint Aims to Overtake ... [https://applyingai.com/2025/09/chinas-state-backed-bci-blueprint-aims-to-overtake-neuralink-by-2027/]
- 102. China's Brain-Computer Interface Industry: Investing in the ... [https://www.china-briefing.com/news/chinas-brain-computer-interface-industry-tapping-into-the-future-of-human-machine-integration/]
- 103. China's new roadmap aims for global BCI leadership by 2030 [https://www.notebookcheck.net/China-s-new-roadmap-aims-for-global-BCI-leadership-by-2030.1102630.0.html]