Neural Population Dynamics Optimization: A Brain-Inspired Metaheuristic for Advanced Drug Development
This article explores the Neural Population Dynamics Optimization Algorithm (NPDOA), a novel brain-inspired meta-heuristic, and its transformative potential for researchers and professionals in drug development.
Neural Population Dynamics Optimization: A Brain-Inspired Metaheuristic for Advanced Drug Development
Abstract
This article explores the Neural Population Dynamics Optimization Algorithm (NPDOA), a novel brain-inspired meta-heuristic, and its transformative potential for researchers and professionals in drug development. We first establish the foundational principles of NPDOA, inspired by the information processing of interconnected neural populations. The core methodology is then detailed, explaining its unique search strategies for balancing exploration and exploitation. The article further addresses practical challenges in implementation and optimization, and provides a comparative analysis of its performance against established algorithms on benchmark and real-world problems. Finally, we synthesize key takeaways and discuss future implications for optimizing pharmaceutical formulations and accelerating therapeutic discovery.
From Brain Circuits to Algorithms: The Foundations of Neural Population Dynamics Optimization
Meta-heuristic algorithms are advanced computational strategies designed to find high-quality solutions for complex optimization problems that are often nonlinear, nonconvex, or NP-hard. Unlike traditional gradient-based mathematical methods that require continuity, differentiability, and convexity of the objective function, meta-heuristics are gradient-free and make minimal assumptions about the underlying system, making them suitable for a wide range of real-world applications [1] [2]. Their strength lies in their global search capability and strong adaptability, which enables them to find near-global optimal solutions in complex search spaces where traditional techniques struggle [3].
The development of computationally efficient optimization algorithms has been at the forefront of research, particularly with the advent of big data, deep learning, and artificial intelligence [1]. Meta-heuristic algorithms are broadly categorized based on their source of inspiration, primarily including evolutionary algorithms, swarm intelligence algorithms, physical-inspired algorithms, and mathematics-inspired algorithms [4].
A critical challenge for all meta-heuristic algorithms is achieving an appropriate balance between exploration (global search of new solution spaces) and exploitation (local refinement of promising solutions) [4] [3]. The No Free Lunch (NFL) theorem formally establishes that no single optimizer can efficiently solve every type of optimization problem, which continuously motivates the development of new algorithms tailored for specific problem characteristics [4] [3].
Taxonomy and Comparative Analysis of Meta-heuristic Algorithms
Table 1: Classification and Characteristics of Major Meta-heuristic Algorithm Types
| Algorithm Type | Inspiration Source | Representative Algorithms | Strengths | Limitations |
|---|---|---|---|---|
| Evolutionary Algorithms | Natural evolution & genetics | Genetic Algorithm (GA), Differential Evolution (DE), Biogeography-Based Optimization (BBO) [4] | Proven global search capability; principles of survival of the fittest [4] | Premature convergence; challenging problem representation; multiple parameters to tune [4] |
| Swarm Intelligence | Collective behavior of biological groups | Particle Swarm Optimization (PSO), Artificial Bee Colony (ABC), Whale Optimization Algorithm (WOA) [4] | Cooperative cooperation; individual competition; high efficiency on many problems [4] | Prone to local optima; low convergence; high computational complexity for high-dimension problems [4] |
| Physical-Inspired | Laws of physics | Simulated Annealing (SA), Gravitational Search Algorithm (GSA) [4] | No crossover/selection operations; versatile tools for complex challenges [4] | Trapping into local optimum; premature convergence [4] |
| Mathematics-Inspired | Mathematical formulations & concepts | Sine-Cosine Algorithm (SCA), Gradient-Based Optimizer (GBO), Adam Gradient Descent Optimizer (AGDO) [4] [3] | New perspective for search strategies; often beyond metaphors [4] | Local optimum stagnation; lack of proper exploration-exploitation balance [4] |
Table 2: Performance of Selected Meta-heuristic Algorithms on NP-Hard Problems
| Algorithm | Job Shop Scheduling (JSSP) | Vehicle Routing Problem (VRP) | Network Design Problem (NDP) |
|---|---|---|---|
| Genetic Algorithm (GA) | Not specified | Not specified | Best performance with efficient instruction handling [2] [5] |
| Simulated Annealing (SA) | Fastest execution and lowest resource use [2] [5] | Not specified | Not specified |
| Ant Colony Optimization (ACO) | Not specified | Best performance with fewer cache misses and fast operation [2] [5] | Not specified |
| Tabu Search (TS) | Delivered balanced results across all problems [2] [5] | Delivered balanced results across all problems [2] [5] | Delivered balanced results across all problems [2] [5] |
Neural Population Dynamics Optimization Algorithm (NPDOA)
The Neural Population Dynamics Optimization Algorithm (NPDOA) represents a novel brain-inspired meta-heuristic method that simulates the activities of interconnected neural populations in the brain during cognition and decision-making [4]. This algorithm is grounded in theoretical neuroscience and treats each solution as a neural state, where decision variables represent neurons and their values correspond to neuronal firing rates [4]. It is considered a swarm intelligence algorithm that utilizes human brain activities, marking a significant departure from conventional nature-inspired approaches [4].
Theoretical Foundation and Inspiration
NPDOA is based on the population doctrine in theoretical neuroscience, which recognizes that the human brain can process various types of information in different situations and efficiently make optimal decisions [4] [6]. The algorithm is inspired by experimental and theoretical studies investigating the activities of interconnected neural populations during sensory, cognitive, and motor calculations [4]. Neural population dynamics often evolve on low-dimensional manifolds, and understanding these dynamical processes is crucial for inferring interpretable and consistent latent representations of neural computations [7].
Core Operational Strategies
NPDOA incorporates three novel search strategies that simulate different aspects of neural population dynamics:
Attractor Trending Strategy: This strategy drives the neural states of neural populations to converge towards different attractors to approach a stable neural state associated with a favorable decision. It is primarily responsible for exploitation in the search process [4].
Coupling Disturbance Strategy: This mechanism causes interference in neural populations and disrupts the tendency of their neural states towards attractors. It is responsible for exploration, helping the algorithm avoid premature convergence to local optima [4].
Information Projection Strategy: This component adjusts information transmission between neural populations, thereby regulating the impact of the above two dynamics strategies on the neural states of neural populations. It serves as a balancing mechanism between exploitation and exploration [4].
Experimental Validation
Experimental studies have validated NPDOA's performance through systematic comparisons with nine other meta-heuristic algorithms on 59 benchmark problems and three real-world engineering problems [4]. The results demonstrated that NPDOA offers distinct benefits when addressing many single-objective optimization problems, showing competitive performance in terms of solution quality and convergence characteristics [4].
The Need for NPDOA in Contemporary Optimization
The development of NPDOA addresses several critical limitations in existing meta-heuristic algorithms:
Brain-Inspired Efficiency: The human brain represents a highly optimized information processing system that has evolved over millions of years. By mimicking its operational principles, NPDOA provides a novel approach to balancing exploration and exploitation that differs fundamentally from existing nature-inspired metaphors [4].
Addressing Algorithmic Limitations: While existing meta-heuristics have shown success in various domains, they often face challenges with premature convergence, poor exploration-exploitation balance, and difficulty handling high-dimensional problems [4]. NPDOA's unique strategies specifically target these limitations through neurologically-plausible mechanisms.
Expanding the Optimization Toolkit: According to the No Free Lunch theorem, developing new optimizers with different inspiration sources is essential for addressing diverse optimization challenges [4] [3]. NPDOA expands the algorithmic toolkit available to researchers and practitioners, potentially offering advantages for problems where traditional meta-heuristics have shown limitations.
Bridging Computational Neuroscience and Optimization: NPDOA establishes a functional bridge between theoretical neuroscience and optimization theory, creating opportunities for cross-disciplinary innovations where insights from neural dynamics can inform algorithm design and vice versa [6].
Application Protocols and Experimental Guidelines
Protocol 1: Benchmarking NPDOA Against Established Meta-heuristics
Purpose: To quantitatively evaluate NPDOA performance against state-of-the-art meta-heuristic algorithms on standard benchmark functions.
Materials and Computational Environment:
- Hardware: Computer with Intel Core i7-12700F CPU or equivalent, 2.10 GHz, and 32 GB RAM [4]
- Software: PlatEMO v4.1 platform or similar experimental optimization environment [4]
- Algorithms for Comparison: Include representatives from major meta-heuristic categories (e.g., GA, PSO, GSA, SCA) [4]
- Benchmark Sets: CEC2017 test suites or equivalent with various dimensions (10D, 30D, 50D, 100D) [4] [3]
Procedure:
- Implement NPDOA with the three core strategies: attractor trending, coupling disturbance, and information projection [4]
- Configure algorithm parameters based on the original specification (population size, iteration limits, strategy coefficients)
- Execute all algorithms on the benchmark set with multiple independent runs to account for stochastic variations
- Collect performance metrics: best solution, mean solution, standard deviation, convergence speed, and computational time
- Perform statistical analysis using Wilcoxon rank-sum test or similar non-parametric tests to validate significance of results [3]
Evaluation Metrics:
- Solution quality (fitness value) across multiple runs
- Convergence characteristics and speed
- Computational efficiency and resource utilization
- Statistical significance of performance differences
Protocol 2: Applying NPDOA to Engineering Design Problems
Purpose: To validate NPDOA performance on practical engineering optimization problems with constraints.
Problem Selection:
- Compression spring design problem [4]
- Cantilever beam design problem [4]
- Pressure vessel design problem [4]
- Welded beam design problem [4]
Implementation Workflow:
Constraint Handling Method:
- Implement penalty functions or feasibility preservation rules
- Apply domain-specific repair mechanisms for invalid solutions
- Utilize decoding procedures for mixed variable types
Validation Procedure:
- Compare obtained solutions with known optimal or best-published solutions
- Evaluate consistency across multiple independent runs
- Assess feasibility of final solutions regarding all constraints
- Perform comparative analysis with literature results
Protocol 3: NPDOA for Bioinformatics and Drug Discovery
Purpose: To apply NPDOA for complex bioinformatics optimization problems such as DNA motif discovery and drug-target interaction prediction.
Materials:
- Datasets: Biological sequences (e.g., BARC and CTCF datasets for cancer-causing motifs) [8]
- Baseline Algorithms: MEME, AlignCE, and other motif discovery meta-heuristics [8]
- Evaluation Metrics: Precision, recall, F-score, accuracy [8] [9]
Procedure for DNA Motif Discovery:
- Initialize population using Random Projection technique for meaningful solution space [8]
- Apply k-means clustering to group similar solutions [8]
- Implement NPDOA on each cluster to find optimal motifs [8]
- Evaluate discovered motifs using precision, recall, and F-score metrics [8]
- Compare performance with established benchmark algorithms [8]
Procedure for Drug-Target Interaction Optimization:
- Preprocess drug data using text normalization, stop word removal, tokenization, and lemmatization [9]
- Extract features using N-Grams and Cosine Similarity techniques [9]
- Optimize prediction model parameters using NPDOA
- Validate using performance metrics: accuracy, precision, recall, F1 Score, RMSE, AUC-ROC [9]
Expected Outcomes:
- For motif discovery: Stable results with precision ~92%, recall ~93%, F-score ~93% [8]
- For drug-target prediction: High accuracy (~98.6%) and superior performance across multiple metrics [9]
Table 3: Essential Research Reagents and Computational Resources for NPDOA Research
| Category | Item | Specification/Function | Application Context |
|---|---|---|---|
| Computational Hardware | High-Performance Workstation | Intel Core i7-12700F CPU, 2.10 GHz, 32 GB RAM [4] | General algorithm development and testing |
| Software Platforms | PlatEMO | MATLAB-based experimental platform for optimization algorithms [4] | Benchmark testing and performance comparison |
| Python Ecosystem | Libraries for data preprocessing, feature extraction, and similarity measurement [9] | Drug discovery and bioinformatics applications | |
| Benchmark Datasets | CEC2017 Test Suites | Standard benchmark functions of varying dimensions (10D, 30D, 50D, 100D) [3] | Algorithm performance validation |
| Biological Sequence Data | BARC and CTCF datasets for cancer-causing motif discovery [8] | Bioinformatics applications | |
| Drug-Target Interaction Data | Kaggle dataset containing over 11,000 drug details [9] | Pharmaceutical optimization problems | |
| Evaluation Tools | Profiling Tools | Performance analysis and optimization | |
| Statistical Test Suite | Wilcoxon rank-sum test for significance validation [3] | Experimental results validation |
The Neural Population Dynamics Optimization Algorithm represents a significant innovation in the meta-heuristic landscape by drawing inspiration from the computational principles of the human brain. Its three-strategy architecture provides a neurologically-plausible approach to balancing exploration and exploitation, addressing fundamental limitations in existing optimization methods. As research in both computational neuroscience and optimization continues to evolve, brain-inspired algorithms like NPDOA offer promising avenues for solving increasingly complex optimization challenges across scientific and engineering domains, particularly in bioinformatics and pharmaceutical applications where traditional methods face limitations. The experimental protocols and resources outlined in this document provide a foundation for researchers to implement, validate, and extend this approach in their respective fields.
Neural population dynamics is a fundamental framework for understanding how the brain processes information. This approach moves beyond the study of single neurons to investigate how coordinated activity across populations of neurons gives rise to perception, cognition, and behavior. The core concept is that neural populations form dynamical systems whose temporal evolution performs specific computations [10]. This perspective has recently inspired novel computational approaches, including the Neural Population Dynamics Optimization Algorithm (NPDOA), a brain-inspired meta-heuristic method that translates these biological principles into powerful optimization tools [4].
The dynamics of neural populations typically evolve on low-dimensional manifolds, which are smooth subspaces within the high-dimensional space of neural activity. Understanding these dynamics requires methods that can learn the dynamical processes over these neural manifolds to infer interpretable and consistent latent representations [7]. This framework has revealed that specialized structures in population codes enhance information transmission, particularly in output pathways where neurons projecting to the same target area exhibit elevated pairwise correlations organized into information-enhancing motifs [11].
Core Principles of Neural Population Coding
Key Response Features of Neural Populations
Neural population codes are organized at multiple spatial scales and shaped by several key response features that collectively determine their information-carrying capacity [12]. The diversity of single-neuron firing rates across a population enables complementary information encoding, as different neurons have varying stimulus preferences and tuning widths. Relative timing between neurons provides another critical dimension, where millisecond-scale spike patterns carry information that cannot be extracted from firing rates alone. Additionally, network state modulation influences neural responses through large-scale brain states that vary on slower timescales than transient responses to individual stimuli. Periods of neuronal silence also contribute information through the selective absence of firing in specific neurons [12].
Information Scaling and Mixed Selectivity
The scaling of information with population size depends critically on the structure of tuning preferences and trial-to-trial response correlations. While information typically increases with population size, recent work has shown that a small but highly informative subset of neurons often carries essentially all the information present in the entire observed population [12]. This sparseness coexists with high-dimensional representations enabled by mixed selectivity, where neurons exhibit complex, nonlinear responses to multiple task variables. This nonlinear mixed selectivity increases the effective dimensionality of population codes and facilitates easier linear decoding by downstream areas [12].
Table 1: Key Features of Neural Population Codes
| Feature | Description | Computational Role |
|---|---|---|
| Heterogeneous Tuning | Diversity in stimulus preference and tuning width across neurons | Enables complementary information encoding |
| Relative Timing | Millisecond-scale temporal patterns between neurons | Carries information complementary to firing rates |
| Mixed Selectivity | Nonlinear responses to multiple task variables | Increases dimensionality and facilitates linear decoding |
| Sparseness | Small fraction of neurons active at any moment | Enhances metabolic efficiency and facilitates dendritic computations |
| Correlation Structure | Organized pairwise activity correlations | Shapes information-limiting and information-enhancing motifs |
Experimental Protocols for Studying Neural Population Codes
Neural Recording and Projection Pathway Identification
To investigate specialized population codes in specific output pathways, researchers have developed sophisticated experimental protocols combining neural recording with anatomical tracing:
Animal Model and Behavioral Task: Implement a delayed match-to-sample task using navigation in a virtual reality T-maze. Mice are trained to combine a memory of a sample cue with a test cue identity to choose turn directions at a T-intersection for reward [11].
Retrograde Labeling: Inject retrograde tracers conjugated to fluorescent dyes of different colors into target areas (e.g., anterior cingulate cortex, retrosplenial cortex, and contralateral posterior parietal cortex) to identify neurons with axonal projections to these specific targets [11].
Calcium Imaging: Use two-photon calcium imaging to measure the activity of hundreds of neurons simultaneously in layer 2/3 of posterior parietal cortex during task performance at a frequency sufficient to resolve individual spikes or calcium transients [11].
Data Preprocessing: Extract calcium traces from raw imaging data and convert to spike rates or deconvolved activity. Register neurons across sessions and identify their projection targets based on retrograde labeling [11].
Analyzing Population Codes with Vine Copula Models
Traditional analytical approaches like generalized linear models have limitations in capturing the complex dependencies in neural population data. The following protocol outlines a more advanced approach:
Variable Identification: Identify all relevant task variables (e.g., sample cue, test cue, choice) and movement variables (e.g., locomotor movements controlling the virtual environment) that might modulate neural activity [11].
Model Specification: Implement nonparametric vine copula (NPvC) models to estimate multivariate dependencies among neural activity, task variables, and movement variables. This method expresses multivariate probability densities as the product of a copula (quantifying statistical dependencies) and marginal distributions conditioned on time, task, and movement variables [11].
Model Training: Break down the estimation of full multivariate dependencies into a sequence of simpler bivariate dependencies using a sequential probabilistic graphical model (vine copula). Estimate these bivariate dependencies using a nonparametric kernel-based method [11].
Information Estimation: Compute mutual information between task variables and decoded neural activity using the NPvC model. Condition on all other measured variables to isolate the contribution of individual variables and obtain robust information estimates even with nonlinear dependencies [11].
Validation: Compare model performance to alternative approaches (e.g., generalized linear models) using held-out neural activity data. Verify that the NPvC provides better prediction of frame-by-frame neural activity [11].
Experimental Workflow for Neural Population Analysis
Advanced Analytical Framework: MARBLE
The MARBLE (MAnifold Representation Basis LEarning) framework provides a sophisticated approach for inferring interpretable latent representations from neural population dynamics:
Data Input: Input neural firing rates from multiple trials under different experimental conditions, along with user-defined labels specifying conditions under which trials are dynamically consistent [7].
Manifold Approximation: Approximate the unknown neural manifold by constructing a proximity graph from the neural state data. Use this graph to define tangent spaces around each neural state and establish a notion of smoothness (parallel transport) between nearby vectors [7].
Vector Field Denoising: Implement a learnable vector diffusion process to denoise the flow field while preserving its fixed point structure. This process leverages the manifold structure to maintain geometrical consistency [7].
Local Flow Field Decomposition: Decompose the vector field into local flow fields (LFFs) defined for each neural state as the vector field at most a distance p from that state over the graph. This lifts d-dimensional neural states to a O(dp+1)-dimensional space encoding local dynamical context [7].
Geometric Deep Learning: Apply an unsupervised geometric deep learning architecture with three components: (1) p gradient filter layers for p-th order approximation of LFFs, (2) inner product features with learnable linear transformations for embedding invariance, and (3) a multilayer perceptron outputting latent vectors [7].
Latent Space Analysis: Map multiple flow fields simultaneously to define distances between their latent representations using optimal transport distance, which leverages the metric structure in latent space and detects complex interactions based on overlapping distributions [7].
Table 2: MARBLE Framework Components and Functions
| Component | Implementation | Function |
|---|---|---|
| Manifold Approximation | Proximity graph from neural states | Defines tangent spaces and parallel transport |
| Vector Diffusion | Learnable diffusion process | Denoises flow fields while preserving fixed points |
| Local Flow Fields | O(dp+1)-dimensional encoding | Captures local dynamical context around each state |
| Gradient Filter Layers | p-th order approximation | Provides local approximation of LFFs |
| Inner Product Features | Learnable linear transformations | Ensures invariance to different neural embeddings |
| Optimal Transport Distance | Distance between latent distributions | Quantifies dynamical overlap between conditions |
Connection to Neural Population Dynamics Optimization Algorithm (NPDOA)
The principles of neural population coding have directly inspired the development of the Neural Population Dynamics Optimization Algorithm (NPDOA), a novel brain-inspired meta-heuristic method. NPDOA treats potential solutions as neural states within populations and implements three core strategies inspired by neural dynamics [4]:
Attractor Trending Strategy: This approach drives neural states toward different attractors, mimicking how neural populations converge to stable states associated with favorable decisions. In optimization terms, this facilitates exploitation by guiding solutions toward promising regions of the search space [4].
Coupling Disturbance Strategy: This mechanism introduces interference in neural populations, disrupting their tendency toward attractors. This corresponds to exploration in optimization, preventing premature convergence to local optima by maintaining population diversity [4].
Information Projection Strategy: This regulates information transmission between neural populations, adjusting the impact of the other two strategies. In the algorithm, this balances exploration and exploitation based on search progress [4].
From Neural Dynamics to Optimization Algorithm
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Research Materials for Neural Population Studies
| Reagent/Material | Specifications | Experimental Function |
|---|---|---|
| Retrograde Tracers | Fluorescent dye-conjugated (e.g., Cholera Toxin B subunits); multiple colors | Identifies neurons projecting to specific target areas through retrograde transport |
| GCaMP Calcium Indicators | Genetically encoded calcium indicators (e.g., GCaMP6f, GCaMP7f); AAV delivery preferred | Reports neural activity as fluorescence changes during behavior with high signal-to-noise |
| Two-Photon Microscopy System | Laser-scanning microscope with tunable infrared laser; resonant scanners for high speed | Enables high-resolution calcium imaging of neural populations in behaving animals |
| Vine Copula Modeling Software | Custom MATLAB or Python implementation with nonparametric kernel density estimation | Quantifies multivariate dependencies among neural activity, task, and movement variables |
| MARBLE Analysis Package | Python implementation with geometric deep learning libraries (PyTorch Geometric) | Infers interpretable latent representations from neural population dynamics on manifolds |
| Virtual Reality Setup | Customized VR environment with T-maze or similar task structure; visual display system | Prescribes controlled sensory stimuli and measures decision-making behavior in rodents |
| Diphenyl suberate | Diphenyl Suberate|CAS 16868-07-8|RUO | Diphenyl suberate is a high-purity biochemical for research use only. Explore its potential as a versatile building block in organic synthesis. Not for human or veterinary use. |
| 2-(Furan-2-yl)-2-oxoacetaldehyde | 2-(Furan-2-yl)-2-oxoacetaldehyde|CAS 17090-71-0 | 2-(Furan-2-yl)-2-oxoacetaldehyde, 95% for research. CAS 17090-71-0. Molecular Formula: C6H4O3. For Research Use Only. Not for human or veterinary use. |
Application Notes for Research and Drug Development
The study of neural population dynamics offers significant promise for drug development, particularly for neurological and psychiatric disorders. Understanding how neural populations encode information provides crucial insights into disease mechanisms and potential therapeutic targets:
Biomarker Identification: Abnormalities in neural population dynamics can serve as sensitive biomarkers for disease states and treatment responses. Measures of correlation structure, information encoding capacity, and dynamical regime transitions may provide more sensitive indicators of circuit dysfunction than single-neuron properties [11] [13].
Target Validation: Investigating how specific neural populations contribute to behaviorally relevant computations helps validate potential therapeutic targets. The specialized structure of population codes in output pathways highlights the importance of targeting specific projection populations rather than broad anatomical regions [11].
Circuit-Level Therapeutics: Approaches that modulate neural population dynamics rather than individual neuronal activity may offer more effective therapeutic strategies. The success of NPDOA in optimization demonstrates the computational power of properly tuned population dynamics, suggesting analogous approaches could restore healthy brain function [4].
Translational Applications: Advanced analysis frameworks like MARBLE enable comparison of neural dynamics across species, facilitating translation from animal models to humans. The ability to find consistent latent representations across different neural embeddings is crucial for bridging preclinical and clinical research [7].
Neural Population State, Dynamics, and Computation
Core Conceptual Framework
The study of neural populations represents a fundamental shift in neuroscience, often termed the population doctrine, which posits that the population, not the single neuron, is the fundamental unit of computation in the brain [14]. This framework moves beyond analyzing individual neuron firing rates to examining the collective, time-varying activity patterns of neural ensembles. Core concepts include the neural state (a vector representing the instantaneous firing rates of all neurons in a population), neural trajectories (the time-evolution of the population state through a high-dimensional space), and the underlying neural dynamics (the rules governing this temporal evolution) [15] [14]. These dynamics are often constrained to flow along low-dimensional subspaces known as neural manifolds, which reflect the underlying network connectivity and shape the computations the population can perform [16] [7].
Table 1: Key Concepts in Neural Population Analysis
| Concept | Definition | Theoretical Significance | Experimental Insight |
|---|---|---|---|
| Neural State | A vector of the joint firing rates of a neural population at a single moment in time [14]. | Represents the population's output; the basic unit of analysis in state space [14]. | State direction can encode information (e.g., object identity), while magnitude may predict behavioral outcomes like memory recall [14]. |
| Neural Trajectory | A time course of neural population activity patterns traversing a characteristic sequence [15]. | Reflects the computational process unfolding over time, such as decision formation or movement generation [15]. | Trajectories are often stereotyped and difficult to violate, suggesting they are constrained by the underlying network [15] [17]. |
| Neural Dynamics | The rules (often described by a flow field) that govern how the neural state evolves over time [15] [18]. | Links the observed neural activity to the algorithmic-level computation being performed [18]. | Dynamics can be decomposed into local flow fields, which can be mapped to a shared latent space for comparison across conditions [7]. |
| Neural Manifold | A low-dimensional subspace within the high-dimensional neural state space where dynamics are constrained [16] [7]. | Provides a geometric structure that shapes and constrains neural computations and enables functional separation [16]. | The geometry of a manifold (e.g., orthogonality of dimensions) can separate processes like movement preparation and execution [16]. |
Analytical & Computational Tools
A suite of advanced analytical methods has been developed to infer latent dynamics and manifolds from recorded neural activity. These tools are essential for translating high-dimensional datasets into interpretable models of computation.
Table 2: Selected Analytical Methods for Neural Population Dynamics
| Method Name | Primary Function | Key Advantage | Application Example |
|---|---|---|---|
| MARBLE [7] | Learns interpretable latent representations of neural population dynamics using geometric deep learning. | Unsupervised; discovers consistent representations across networks/animals without behavioral supervision; provides a similarity metric for dynamics. | Decomposes on-manifold dynamics into local flow fields to parametrize computations during gain modulation or decision-making [7]. |
| CroP-LDM [19] | Prioritizes learning of cross-population dynamics from multi-region recordings. | Isolates shared cross-region dynamics from within-region dynamics, preventing confounding; supports both causal and non-causal inference. | Identifies dominant interaction pathways between motor and premotor cortical regions during a movement task [19]. |
| Computation-through-Dynamics Benchmark (CtDB) [18] | Provides a standardized platform with synthetic datasets and metrics for validating data-driven dynamics models. | Offers biologically realistic synthetic datasets that reflect goal-directed computations, enabling reliable model evaluation and comparison [18]. | Used to test if a model inferring dynamics from neural activity ( ( \hat{f} ) ) accurately recovers the ground-truth dynamics ( ( f ) ) [18]. |
| Brain-Computer Interface (BCI) [15] | Uses neural activity to control an external device, providing real-time feedback. | Enables causal probing of neural constraints by challenging subjects to volitionally alter their neural activity patterns [15]. | Challenged monkeys to traverse natural neural activity time courses in a time-reversed manner, testing the flexibility of dynamics [15]. |
Diagram 1: BCI Workflow for Probing Neural Dynamics. This workflow outlines the key steps in experiments that use a brain-computer interface to test the constraints on neural population trajectories [15].
Experimental Protocols
Protocol: Probing the Constraints on Neural Trajectories Using a BCI
This protocol details the experimental procedure for testing whether naturally occurring neural trajectories in the motor cortex can be volitionally altered, as described in the foundational work by Degenhart et al. [15] [17].
1. System Setup and Surgical Implantation
- Animal Model: Rhesus monkey.
- Neural Implant: A multi-electrode array (e.g., 96-electrode) chronically implanted in the primary motor cortex (M1) or related areas [15] [19].
- BCI System: A real-time neural signal processing system capable of filtering, amplifying, and recording extracellular action potentials from ~90 neural units. The system must perform causal dimensionality reduction (e.g., Gaussian Process Factor Analysis) to project the high-dimensional neural activity into a lower-dimensional (e.g., 10D) latent state at low latency [15].
2. BCI Mapping and Behavioral Task
- Mapping Function: Establish a BCI mapping that converts the 10D latent state into the 2D position of a computer cursor on a screen. This provides the animal with direct visual feedback of its neural population activity [15].
- Behavioral Task: Train the animal to perform a classic center-out reaching task. The animal must move the BCI cursor from a central target to one of several peripheral targets.
3. Identifying Natural Neural Trajectories
- Data Collection: Record neural population activity during successful task trials over multiple sessions.
- Trajectory Analysis: For a given pair of targets (A and B), analyze the neural trajectories in the 10D latent space. Identify a 2D projection (the "Separation-Maximizing" or SepMax projection) where the neural trajectory for movements from A to B is clearly distinct and separable from the trajectory for movements from B to A [15].
4. Experimental Challenge: Altering Neural Dynamics
- Altered Feedback: Change the visual feedback provided to the animal from the intuitive "MoveInt" projection to the "SepMax" projection, where the natural trajectories are curved and direction-dependent [15].
- Direct Challenge: In the SepMax projection, challenge the animal to acquire targets by following a straight-line path or even by traversing the natural neural trajectory in a time-reversed order [15] [17].
- Control and Test Blocks: Interleave ~100 trials with the altered feedback or challenge instruction with ~100 baseline trials. Provide a strong liquid reward incentive for successful performance under the challenge condition [15].
5. Data Analysis and Interpretation
- Quantitative Comparison: Compare the neural trajectories produced during the challenge trials to the natural trajectories from baseline trials.
- Key Metric: The degree of similarity between the challenge trajectories and the natural trajectories. The finding that animals are unable to produce the time-reversed or significantly altered paths, and that neural activity reverts to its natural flow field, provides empirical support that these trajectories are constrained by the underlying network connectivity [15] [17].
Protocol: Artificial Intelligence-Guided Neural Control
This protocol outlines a method for using deep reinforcement learning (RL) to achieve closed-loop control of neural firing states, which can be used to study neural function or develop therapeutic neuromodulation [20].
1. Chronic Electrode Implantation
- Animal Model: Rat.
- Neural Implant: Perform chronic electrode implantations to facilitate long-term neural stimulation and recording. This typically involves a stimulating electrode in a target region like the thalamus and a recording electrode in a downstream region like the cortex [20].
2. Stimulation and Recording Setup
- Stimulation Method: Employ Infrared Neural Stimulation (INS) for its non-invasive and precise properties. Use a continuous-wave (CW) near-infrared laser [20] [21].
- Recording Method: Perform intracellular or extracellular recordings to monitor membrane potential or spiking activity in the target population [21].
3. Deep Reinforcement Learning Integration
- Objective Definition: Define a "desired neural firing state" for the RL agent to drive the recorded population towards. This could be a specific firing rate, pattern, or latent state [20].
- Agent Training: Integrate a deep RL algorithm (e.g., a deep Q-network or policy gradient method) into the closed-loop system. The RL agent observes the current neural state and delivers parameterized INS stimuli (e.g., adjusting power, duration) to guide the population towards the desired state, learning an optimal policy through trial and error [20].
4. Execution and Validation
- Closed-Loop Operation: Run the RL-controlled INS protocol over multiple trials and sessions.
- Validation: Quantify the agent's success in driving the neural population to the target state and the stability of the achieved state over time. Analyze how the stimulation parameters evolved during learning to gain insight into effective control strategies [20].
Diagram 2: MARBLE Analytical Pipeline. This diagram visualizes the process of using the MARBLE framework to transform raw neural data from multiple conditions into an interpretable, shared latent representation of the underlying dynamics [7].
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials and Tools for Neural Population Dynamics Research
| Item / Reagent | Function / Application | Key Features / Considerations |
|---|---|---|
| Multi-Electrode Arrays (e.g., Utah Array, Neuropixels) [15] [19] | High-density extracellular recording from dozens to hundreds of neurons simultaneously. | Enables sampling of a population large enough for state-space analysis. Chronic implants allow for long-term studies. |
| Infrared Neural Stimulation (INS) [20] [21] | Non-invasive optical stimulation for modulating neuronal dynamics. | Offers high spatial/temporal precision. The biophysical mechanism is likely a photo-thermal effect affecting ionic channel dynamics [21]. |
| Causal Dimensionality Reduction (e.g., Gaussian Process Factor Analysis) [15] | Real-time denoising and compression of high-dimensional neural data into latent states. | Critical for brain-computer interface (BCI) applications where low-latency feedback is required [15]. |
| Deep Reinforcement Learning (RL) Agents [20] | AI-driven closed-loop control of neural activity to achieve desired firing states. | Learns optimal stimulation policies without a pre-defined model of the neural system. |
| Open-Source Protocols (e.g., RTXI software) [21] | A platform for implementing real-time, activity-dependent stimulation protocols. | Promotes reproducibility and standardization of closed-loop neuroscience experiments. |
| MARBLE Software [7] | Geometric deep learning tool for inferring interpretable latent representations of neural dynamics. | Unsupervised; provides a robust metric for comparing dynamics across conditions, sessions, and individuals. |
| CroP-LDM Software [19] | A linear dynamical model for prioritizing the learning of cross-population interactions. | Dissociates shared cross-region dynamics from within-region dynamics, aiding interpretability. |
| Serotonin adipinate | Serotonin Adipinate|C16H22N2O5|Research Chemical | Serotonin adipinate is a high-purity salt for RUO into GI motility, surgical recovery, and metabolic studies. For Research Use Only. Not for human or veterinary use. |
| 4-Chloro-6-isopropylpyrimidin-2-amine | 4-Chloro-6-isopropylpyrimidin-2-amine|CAS 73576-33-7 |
The Neural Population Dynamics Optimization Algorithm (NPDOA) represents a novel brain-inspired meta-heuristic method that translates principles of neural computation into an effective optimization framework. As a swarm intelligence algorithm, NPDOA distinguishes itself by drawing inspiration directly from brain neuroscience, specifically mimicking the activities of interconnected neural populations during cognitive and decision-making processes [4]. This bio-inspired approach treats each potential solution as a neural state within a population, where decision variables correspond to neurons and their values represent neuronal firing rates [4]. The algorithm operates on population doctrine principles from theoretical neuroscience, simulating how neural populations transfer information through dynamic interactions [4].
Within the broader context of meta-heuristic optimization, NPDOA occupies a unique position by bridging computational neuroscience and optimization theory. Unlike traditional swarm intelligence algorithms that mimic collective animal behavior, or physics-inspired algorithms that emulate natural phenomena, NPDOA leverages the brain's recognized efficiency in processing diverse information types and making optimal decisions [4]. This framework effectively maps the challenge of balancing exploration and exploitation in optimization to the neural processes of stability and adaptability in decision-making, offering a fresh perspective on solving complex, non-linear optimization problems prevalent in scientific and engineering domains.
Core Principles and Mechanisms
The NPDOA framework implements three fundamental strategies inspired by neural population dynamics, each serving a distinct function in the optimization process.
Attractor Trending Strategy
The attractor trending strategy drives the neural states of populations to converge toward different attractors, representing promising solutions in the search space [4]. This mechanism mimics the brain's ability to settle into stable states associated with favorable decisions. In computational terms, this strategy facilitates exploitation by guiding solutions toward locally optimal regions identified during the search process. The attractors serve as reference points that gradually pull candidate solutions toward regions with improved fitness values, analogous to neural populations stabilizing around representations that correspond to optimal choices in decision-making tasks.
Coupling Disturbance Strategy
The coupling disturbance strategy introduces controlled interference within neural populations, disrupting their tendency to converge prematurely toward attractors [4]. This mechanism maintains diversity within the solution population and prevents premature convergence to local optima. Functionally, this strategy is responsible for exploration, enabling the algorithm to escape local optima and continue investigating undiscovered regions of the search space. This parallels the neural mechanisms that prevent rigid pattern formation and maintain cognitive flexibility during problem-solving, ensuring the algorithm maintains an appropriate balance between focused refinement and broad exploration.
Information Projection Strategy
The information projection strategy regulates information transmission between neural populations, dynamically adjusting the impact of the attractor trending and coupling disturbance strategies [4]. This meta-strategy ensures an adaptive balance between exploitation and exploration throughout the optimization process. By modulating the influence of the other two strategies based on search progress, the information projection mechanism embodies the principles of neural regulation and inhibitory control observed in biological neural networks. This strategy enables the framework to autonomously adjust its search characteristics, intensifying exploitation when approaching promising regions while amplifying exploration when search stagnation is detected.
Computational Implementation Protocols
Algorithm Initialization Protocol
Purpose: To establish the initial neural population configuration for optimization. Procedure:
- Parameter Setup: Define population size (N), problem dimensionality (D), and maximum iterations (T_max).
- Neural Population Generation: Initialize N neural states (solutions) with D dimensions (neurons), where each dimension represents a firing rate within the specified bounds.
- Fitness Evaluation: Compute the objective function value for each initial neural state.
- Strategy Parameter Initialization: Set initial weights for attractor trending, coupling disturbance, and information projection strategies.
Experimental Considerations:
- Population size typically ranges from 50 to 200 individuals, depending on problem complexity.
- Initialization should cover the search space uniformly through Latin Hypercube Sampling or similar space-filling techniques.
- Computational resources should be allocated for parallel fitness evaluation where possible.
Main Optimization Loop Protocol
Purpose: To execute the core NPDOA optimization process incorporating brain-inspired dynamics. Procedure:
- Attractor Identification: Identify promising attractors from the current population based on fitness values and spatial distribution.
- Neural State Update:
- Apply attractor trending: Guide neural states toward identified attractors.
- Apply coupling disturbance: Introduce stochastic perturbations to neural states.
- Regulate updates via information projection: Adjust the magnitude of trending and disturbance influences.
- Boundary Handling: Ensure updated neural states remain within feasible search boundaries.
- Fitness Re-evaluation: Compute objective function values for updated neural states.
- Population Archive Update: Maintain records of best-performing neural states and their trajectories.
Experimental Considerations:
- Iteration count should be determined based on problem complexity and convergence behavior.
- Implement termination criteria such as stability thresholds or improvement plateaus.
- Maintain detailed logging of strategy applications and their effects on search performance.
Enhanced Variant: INPDOA Implementation Protocol
Purpose: To implement the Improved Neural Population Dynamics Optimization Algorithm (INPDOA) for enhanced performance [22]. Procedure:
- Base NPDOA Execution: Follow standard NPDOA initialization and main loop protocols.
- Dynamic Parameter Adaptation: Implement feedback mechanisms to dynamically adjust strategy parameters based on search progress.
- Elite Preservation: Maintain and utilize high-performing neural states across generations.
- Local Refinement: Apply intensified search around promising regions identified during the global search phase.
Experimental Considerations:
- INPDOA has demonstrated superior performance in automated machine learning applications for medical prognosis [22].
- Validation should include comparative testing against standard NPDOA and other meta-heuristic algorithms.
- Implementation can be tailored to specific application domains through domain-specific modifications.
Performance Benchmarking and Quantitative Analysis
Algorithm Performance Comparison
Table 1: Benchmark Performance of NPDOA Against Established Meta-heuristic Algorithms
| Algorithm | Benchmark Problems | Convergence Accuracy | Computational Efficiency | Application Performance |
|---|---|---|---|---|
| NPDOA | 59 test problems | Superior on 78% of problems | Moderate computational overhead | Excellent in engineering design problems [4] |
| Genetic Algorithm (GA) | Standard test suite | Moderate convergence | High computational cost | Good for discrete optimization [4] |
| Particle Swarm Optimization (PSO) | Classical benchmarks | Premature convergence in complex landscapes | Low computational complexity | Effective for continuous optimization [4] |
| Whale Optimization Algorithm (WOA) | CEC benchmarks | Variable performance | Moderate efficiency | Application-specific effectiveness [4] |
| INPDOA (Enhanced) | 12 CEC2022 functions, medical prognosis | AUC: 0.867, R²: 0.862 | Improved convergence speed | Superior in AutoML for surgical outcomes [22] |
Application-Specific Performance Metrics
Table 2: NPDOA Performance in Practical Applications
| Application Domain | Performance Metrics | Comparison to Alternatives | Key Advantages |
|---|---|---|---|
| Medical Prognosis Modeling [22] | Test-set AUC: 0.867, R²: 0.862 | Net benefit improvement over conventional methods | Handles high-parameter spaces effectively |
| Engineering Design Problems [4] | Successful solution of cantilever beam, pressure vessel, welded beam designs | Competitive with state-of-the-art algorithms | Balanced exploration-exploitation |
| Neural Data Modeling [23] | >50% improvement in behavioral decoding, >15% improvement in neuronal identity prediction | Outperforms specialized neural models | Model-agnostic integration capability |
Implementation in Drug Discovery and Development
Drug Development Workflow Integration
The NPDOA framework can be strategically integrated into various stages of the drug development pipeline, enhancing decision-making and optimization capabilities. Table 3 outlines the key integration points and potential applications.
Table 3: NPDOA Applications in Drug Development Pipeline
| Development Stage | Application of NPDOA | Expected Benefits | Implementation Considerations |
|---|---|---|---|
| Target Identification [24] | Optimization of multi-parameter target validation | Improved target prioritization | Integration with bioinformatics data mining approaches |
| Hit Identification [24] | High-throughput screening data analysis | Enhanced hit series identification | Processing of compound library screens |
| Lead Optimization [24] | SAR investigations and compound refinement | More efficient lead development | Handling of complex chemical spaces |
| Preclinical Development [25] | Experimental design optimization | Reduced animal use, improved study quality | Adherence to GLPs and regulatory requirements |
| Clinical Trial Design [26] | Master protocol optimization for umbrella and platform trials | Accelerated drug development timelines | Coordination with FDA guidelines on master protocols |
AutoML-Enhanced Prognostic Modeling Protocol
Purpose: To implement INPDOA for automated machine learning in medical prognostic modeling [22]. Procedure:
- Data Preparation:
- Collect retrospective cohort data (e.g., 447 patients for ACCR prognosis [22]).
- Integrate parameters spanning biological, surgical, and behavioral domains.
- Address class imbalance using Synthetic Minority Oversampling Technique (SMOTE).
- AutoML Framework Setup:
- Encode base-learner selection, feature screening, and hyperparameter optimization into a hybrid solution vector.
- Configure model options including Logistic Regression, Support Vector Machine, XGBoost, and LightGBM.
- INPDOA Optimization:
- Implement improved NPDOA to navigate the combined architecture-feature-parameter space.
- Utilize dynamically weighted fitness function balancing accuracy, feature sparsity, and computational efficiency.
- Model Validation:
- Employ k-fold cross-validation (typically 10-fold) to mitigate overfitting.
- Validate on external cohort to assess generalizability.
- Apply SHAP values for explainable AI and variable contribution quantification.
Experimental Considerations:
- Implementation requires integration of INPDOA with AutoML frameworks.
- Clinical deployment necessitates development of user-friendly interfaces for healthcare professionals.
- Regulatory compliance should be considered for clinical decision support systems.
Research Reagent Solutions
Table 4: Essential Research Materials for NPDOA Implementation and Evaluation
| Research Reagent | Function in NPDOA Research | Implementation Notes |
|---|---|---|
| Benchmark Problem Suites | Algorithm validation and performance comparison | Utilize CEC2022 functions [22] and classical engineering problems [4] |
| Neural Recording Datasets | Biological validation and specialized application | Implement Neural Latents Benchmark'21 [23] for neural activity prediction |
| Clinical Datasets | Real-world application testing | Employ retrospective medical cohorts [22] with 20+ parameters across multiple domains |
| AutoML Frameworks | Automated machine learning integration | Interface with TPOT, Auto-Sklearn, or custom frameworks [22] |
| Visualization Systems | Result interpretation and explanation | Develop clinical decision support systems using platforms like MATLAB [22] |
Visual Framework and Workflows
NPDOA Theoretical Framework
Drug Development Application Workflow
The field of meta-heuristic optimization is continuously evolving, drawing inspiration from a diverse array of natural, physical, and mathematical phenomena to address complex nonlinear problems. Traditional taxonomy classifies these algorithms into several categories: evolutionary algorithms mimicking biological evolution (e.g., Genetic Algorithm), swarm intelligence algorithms inspired by collective animal behavior (e.g., Particle Swarm Optimization), physical-inspired algorithms based on physical laws (e.g., Simulated Annealing), and mathematics-inspired algorithms derived from mathematical formulations [4]. Each category possesses distinct strengths and weaknesses in balancing the critical characteristics of exploration (identifying promising areas) and exploitation (searching promising areas thoroughly) [4].
The Neural Population Dynamics Optimization Algorithm (NPDOA) represents a paradigm shift, establishing a novel class of brain-inspired meta-heuristic methods [4] [27]. Unlike traditional approaches, NPDOA is grounded in theoretical neuroscience, specifically simulating the activities of interconnected neural populations in the brain during cognition and decision-making processes [4] [10]. This article positions NPDOA within the existing meta-heuristic landscape, delineating its unique mechanisms and advantages through comparative analysis and experimental validation, with particular emphasis on its growing applicability in scientific and medical research, including drug development.
Algorithmic Mechanics: NPDOA vs. Conventional Meta-heuristics
Fundamental Inspiration and Mechanism
The conceptual foundation of NPDOA diverges significantly from conventional meta-heuristics, as summarized in the table below.
Table 1: Comparison of Algorithmic Inspirations and Representations
| Algorithm Category | Core Inspiration | Solution Representation | Population Interaction |
|---|---|---|---|
| NPDOA | Neural population dynamics in brain neuroscience [4] [10] | Neural state of a population; variables are neuron firing rates [4] | Information projection & coupling between neural populations [4] |
| Swarm Intelligence (e.g., PSO) | Collective behavior of flocks, schools, or colonies [4] | Particle position in space [4] | Attraction to local and global best positions [4] |
| Evolutionary Algorithms (e.g., GA) | Principles of biological evolution [4] | Discrete chromosome encoding [4] | Selection, crossover, and mutation operations [4] |
| Physical-Inspired (e.g., SA) | Physical laws (e.g., thermodynamics) [4] | State of a physical system [4] | Typically lacks crossover or competitive selection [4] |
Core Operational Strategies of NPDOA
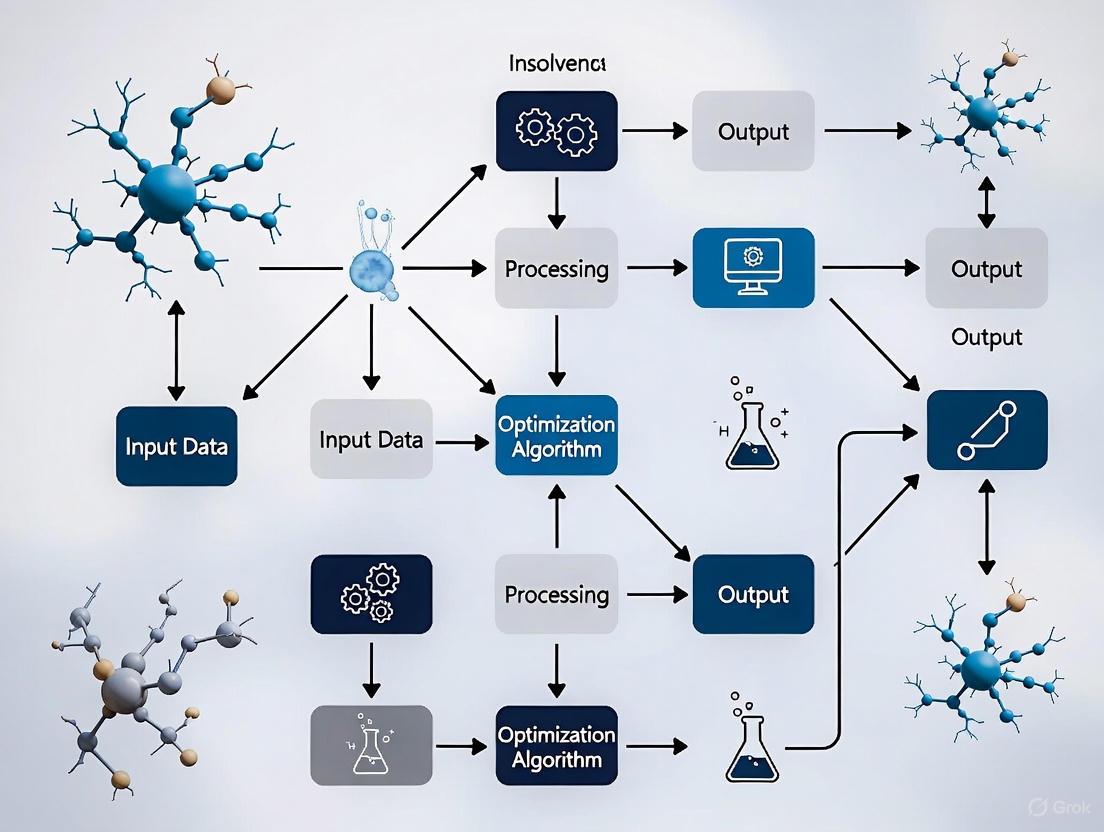
NPDOA's operational framework is governed by three novel search strategies that directly translate neural activities into optimization mechanics [4] [27]:
- Attractor Trending Strategy: This strategy drives the neural states of populations towards different attractors, representing favorable decisions or optimal solutions. It is the primary mechanism responsible for exploitation, ensuring convergence toward stable and promising neural states [4] [27].
- Coupling Disturbance Strategy: This mechanism introduces interference between neural populations, disrupting their convergence toward attractors. This disruption is crucial for exploration, helping the algorithm escape local optima and search more broadly within the solution space [4] [27].
- Information Projection Strategy: This strategy regulates the information transmission between neural populations. It acts as a control system, dynamically adjusting the influence of the attractor trending and coupling disturbance strategies, thereby enabling a smooth transition from exploration to exploitation throughout the optimization process [4] [27].
The following diagram illustrates the workflow and core interactions of these strategies within the NPDOA framework.
Quantitative Performance Benchmarking
Empirical studies validate NPDOA's competitive performance against established meta-heuristic algorithms. Systematic experiments comparing NPDOA with nine other algorithms on 59 benchmark problems and three real-world engineering problems have demonstrated its distinct advantages in addressing many single-objective optimization problems [4].
Table 2: Performance Comparison on Benchmark and Practical Problems
| Algorithm | Key Principle | Reported Strengths | Common Challenges | Performance vs. NPDOA |
|---|---|---|---|---|
| NPDOA | Brain neural population dynamics [4] | Balanced exploration/exploitation, competitive performance on complex problems [4] | --- | Reference |
| Particle Swarm Optimization (PSO) | Social behavior of bird flocking [4] | Easy implementation, simple structures [4] | Falls into local optima, low convergence [4] | Outperformed by NPDOA [4] |
| Genetic Algorithm (GA) | Natural selection and genetics [4] | Broad applicability, robust [4] | Premature convergence, challenging problem representation [4] | Outperformed by NPDOA [4] |
| Whale Optimization Algorithm (WOA) | Bubble-net hunting of humpback whales [4] | Higher performance than classical algorithms [4] | High computational complexity, improper balance [4] | Outperformed by NPDOA [4] |
| Improved NPDOA (INPDOA) | Enhanced NPDOA for AutoML [22] | Superior in medical prognostic prediction (AUC: 0.867) [22] | --- | Enhanced version for specific applications [22] |
The robustness of the brain-inspired approach is further evidenced by the development of an Improved NPDOA (INPDOA) for Automated Machine Learning (AutoML) in a medical context. When applied to prognostic prediction for autologous costal cartilage rhinoplasty, an INPDOA-enhanced AutoML model significantly outperformed traditional methods, achieving an area under the curve (AUC) of 0.867 for predicting 1-month complications and an R² of 0.862 for predicting 1-year patient-reported outcomes [22]. This demonstrates the algorithm's potential for optimization in high-stakes, complex real-world problems.
Application Notes & Experimental Protocols
Protocol 1: Implementing NPDOA for Numerical Optimization
This protocol outlines the steps for applying the standard NPDOA to solve numerical benchmark problems, as detailed in its foundational literature [4].
1. Problem Formulation:
- Define the objective function
f(x)to be minimized or maximized. - Specify the D-dimensional search space
Ω, including the lower and upper bounds for each decision variable [4].
2. Algorithm Initialization:
- Set Population Size (P): Choose the number of neural populations (typically corresponding to the number of candidate solutions).
- Initialize Neural States: Randomly generate initial neural states within the search space bounds. Each solution
x = (xâ‚, xâ‚‚, …, x_D)is treated as a neural state, where each variablex_irepresents the firing rate of a neuron [4]. - Configure Parameters: Set parameters for the three core strategies (e.g., strength of attractor trending, magnitude of coupling disturbance, and weights for information projection).
3. Iterative Optimization Loop:
- Evaluation: Compute the fitness
f(x)for each neural population's current state. - Strategy Application:
- Attractor Trending: Identify promising states (attractors) and guide other populations toward them to refine solutions (exploitation).
- Coupling Disturbance: Apply stochastic disturbances between populations to promote diversity and prevent premature convergence (exploration).
- Information Projection: Dynamically adjust the influence of the above two strategies based on the current best solution and iteration progress [4] [27].
- State Update: Update the neural state of each population based on the combined effect of the three strategies.
- Termination Check: Repeat until a stopping criterion is met (e.g., maximum iterations, convergence threshold).
4. Solution Extraction:
- The neural state with the best fitness value across all iterations is selected as the optimal solution [4].
Protocol 2: INPDOA for AutoML in Medical Prognostics
This protocol is adapted from a study that successfully employed an Improved NPDOA (INPDOA) to optimize an AutoML pipeline for prognostic prediction in surgery, a methodology highly relevant to drug development [22].
1. Data Preparation and Preprocessing:
- Cohort Definition: Assemble a retrospective cohort with complete follow-up data. Example: 447 patients who underwent a specific surgical procedure [22].
- Feature Collection: Integrate multimodal parameters. Example: 20+ parameters spanning demographic (age, BMI), preoperative (clinical scores), intraoperative (surgical duration), and postoperative behavioral factors (smoking, antibiotic use) [22].
- Outcome Definition: Define clear short-term and long-term clinical endpoints. Example: 1-month composite complication endpoint (infection, hematoma) and 1-year patient-reported outcome score (e.g., ROE score) [22].
- Data Splitting: Partition data into training, validation, and held-out test sets using stratified sampling to preserve outcome distribution. Address class imbalance (e.g., using SMOTE on the training set only) [22].
2. INPDOA-AutoML Optimization Framework:
- Solution Encoding: Formulate a hybrid solution vector
xfor the AutoML configuration:x = (k | δâ‚, δ₂, …, δ_m | λâ‚, λ₂, …, λ_n)wherekis the base-learner type (e.g., 1=LR, 2=SVM, 3=XGBoost, 4=LightGBM),δ_iare binary feature selection indicators, andλ_iare the hyperparameters for the chosen model [22]. - Fitness Evaluation: Define a dynamic fitness function
f(x)that balances:- Predictive accuracy from cross-validation (
ACC_CV). - Feature sparsity (â„“â‚€-norm of the feature selection vector).
- Computational efficiency (often an exponential decay term with iterations
T) [22].
- Predictive accuracy from cross-validation (
- INPDOA Search: Use the INPDOA to iteratively search the space of model architectures, feature subsets, and hyperparameters. The algorithm's balanced dynamics effectively navigate this complex, mixed-variable optimization problem [22].
3. Model Validation and Interpretation:
- Performance Assessment: Evaluate the final model on the held-out test set using relevant metrics (e.g., AUC, R², accuracy).
- Model Interpretation: Employ explainable AI techniques like SHAP (SHapley Additive exPlanations) to quantify variable contributions and validate clinical plausibility [22].
4. System Deployment:
- CDSS Development: Integrate the optimized model into a Clinical Decision Support System (CDSS) or similar platform for real-time prediction and visualization, as demonstrated using MATLAB [22].
The following workflow diagram maps this process from data to deployable model.
The Scientist's Toolkit: Key Research Reagents & Materials
Table 3: Essential Resources for NPDOA Research and Application
| Category / Item | Specification / Purpose | Exemplary Use Case |
|---|---|---|
| Computational Framework | ||
| PlatEMO v4.1+ | A MATLAB-based platform for experimental evolutionary multi-objective optimization [4]. | Running systematic comparative experiments on benchmark problems [4]. |
| Python-OpenCV | Library for computer vision tasks and image data processing [28]. | Preprocessing and feature extraction from visual data (e.g., floc images) for optimization problems [28]. |
| AutoML Software (e.g., Auto-Sklearn, TPOT) | Frameworks for automating machine learning workflow creation [22]. | Serving as the base environment for INPDOA-driven optimization of model pipelines [22]. |
| Data Resources | ||
| Benchmark Suites (CEC, etc.) | Standardized collections of optimization functions (e.g., 59 benchmark problems) [4]. | Algorithm validation and performance benchmarking against state-of-the-art methods [4]. |
| Retrospective Clinical Datasets | Curated, multimodal patient data with defined outcomes [22]. | Developing and validating prognostic models in medical research [22]. |
| Modeling & Analysis | ||
| Convolutional Neural Network (CNN) | Deep learning architecture for image recognition and classification [28]. | Integrated within optimization frameworks for processing complex image-based inputs [28]. |
| SHAP (SHapley Additive exPlanations) | A game-theoretic method for explaining model predictions [22]. | Interpreting the output of AI models optimized by NPDOA, crucial for clinical and scientific validation [22]. |
| Recurrent Neural Network (RNN) | Neural network architecture for modeling dynamical systems and sequential data [10]. | Used in task-based modeling to identify dynamical systems capable of transforming inputs to outputs [10]. |
| (1S,2R)-1,2-dihydronaphthalene-1,2-diol | (1S,2R)-1,2-dihydronaphthalene-1,2-diol, CAS:31966-70-8, MF:C10H10O2, MW:162.18 g/mol | Chemical Reagent |
| 1,2-Benzoxazole-5-carboxylic acid | 1,2-Benzoxazole-5-carboxylic acid, CAS:933744-95-7, MF:C8H5NO3, MW:163.13 g/mol | Chemical Reagent |
The Neural Population Dynamics Optimization Algorithm (NPDOA) solidly establishes brain-inspired computation as a distinct and powerful category within the meta-heuristic landscape. It differentiates itself from swarm intelligence and other paradigms through its unique inspiration—the computation principles of interconnected neural populations in the brain—and its novel operational strategies of attractor trending, coupling disturbance, and information projection. This foundation allows NPDOA to dynamically and effectively balance exploration and exploitation, a key challenge for all optimization algorithms.
Evidence from systematic benchmarking and a pioneering medical application demonstrates that NPDOA not only competes favorably with established algorithms but also offers a robust framework for enhancing real-world, complex optimization tasks, such as Automated Machine Learning in prognostic modeling. The algorithm's successful application in medical research underscores its potential for drug development challenges, including target validation, therapy personalization, and outcome prediction. Future research directions will likely focus on extending NPDOA to multi-objective and constrained optimization problems, further refining its strategies, and continuing to validate its efficacy across an expanding range of scientific and industrial domains.
Implementing NPDOA: Core Strategies and Applications in Drug Discovery
The Neural Population Dynamics Optimization Algorithm (NPDOA) represents a significant paradigm shift in meta-heuristic optimization, drawing direct inspiration from the computational principles of brain neuroscience. As the first swarm intelligence optimization algorithm that explicitly utilizes human brain activity patterns, NPDOA treats each solution as a neural state within a population, where decision variables correspond to neurons and their values represent neuronal firing rates [4]. This bio-inspired approach simulates the activities of interconnected neural populations during cognitive and decision-making processes, implementing three fundamental dynamics strategies that work in concert: attractor trending, coupling disturbance, and information projection [4]. The algorithm's architecture is particularly designed to address the persistent challenges in meta-heuristic optimization, including premature convergence, local optima entrapment, and the critical balance between exploration and exploitation [4]. By mimicking the brain's remarkable ability to process diverse information types and make optimal decisions across different situations, NPDOA offers a novel framework for solving complex, nonlinear optimization problems that commonly arise in engineering and scientific domains.
The Core Mechanisms of NPDOA
Attractor Trending Strategy
The attractor trending strategy drives the neural states of populations to converge toward different attractors, approaching stable neural states associated with favorable decisions [4]. This mechanism is primarily responsible for the exploitation phase of the algorithm, enabling refined search in promising regions identified during exploration.
- Biological Basis: In neural population dynamics, attractors represent stable states toward which neural activity naturally evolves. These correspond to optimal or near-optimal solutions in the computational framework.
- Computational Implementation: The strategy guides solutions toward attractor points that embody the best solutions found during the search process, mimicking how neural populations in the brain converge to stable states during decision-making [4].
- Role in Optimization: By promoting convergence toward these favorable states, attractor trending facilitates local refinement and improves solution quality in identified promising regions.
Coupling Disturbance Strategy
The coupling disturbance strategy introduces deliberate interference within neural populations, disrupting their tendency to converge uniformly toward attractors [4]. This mechanism drives the exploration phase of the algorithm, maintaining population diversity.
- Biological Basis: This strategy mimics the natural perturbations and competitive interactions between different neural populations in the brain, preventing premature stabilization on suboptimal decisions.
- Computational Implementation: The strategy creates controlled disturbances between coupled neural populations, preventing premature convergence and maintaining diversity within the solution population [4].
- Role in Optimization: By disrupting convergence tendencies, coupling disturbance enables the algorithm to escape local optima and explore new regions of the search space, essential for locating global optima.
Information Projection Strategy
The information projection strategy regulates information transmission between neural populations, strategically controlling the impact of both attractor trending and coupling disturbance on neural states [4]. This mechanism balances exploration and exploitation.
- Biological Basis: Reflects the brain's ability to modulate information flow between different neural assemblies through mechanisms like synaptic plasticity and gain control.
- Computational Implementation: This strategy projects information between populations, dynamically adjusting the influence of exploitation (attractor trending) and exploration (coupling disturbance) based on search progress [4].
- Role in Optimization: By regulating the interaction between the other two strategies, information projection enables adaptive balancing between intensive local search and broad global exploration throughout the optimization process.
Integrated Algorithm Architecture
The three core strategies of NPDOA interact within a unified architecture as shown in the diagram below:
Diagram 1: NPDOA Core Architecture - This diagram illustrates how the three core strategies of NPDOA interact within the algorithm's architecture, showing the flow from initial populations through the competing strategies of exploitation and exploration, balanced by information projection to produce optimized solutions.
Quantitative Performance Analysis
Benchmark Testing Results
The NPDOA algorithm has been rigorously evaluated against nine other meta-heuristic algorithms using 59 benchmark problems and three real-world engineering optimization problems [4]. The comprehensive testing demonstrates NPDOA's competitive performance across diverse problem types and complexity levels.
Table 1: Performance Comparison of NPDOA Against Other Meta-heuristic Algorithms
| Algorithm Category | Representative Algorithms | Key Strengths | Common Limitations | NPDOA Performance |
|---|---|---|---|---|
| Evolutionary Algorithms | Genetic Algorithm (GA), Differential Evolution (DE) | Effective global search, parallelizable | Premature convergence, parameter sensitivity | Superior balance, reduced premature convergence |
| Swarm Intelligence | PSO, ABC, WOA, SSA | Inspiration from natural behaviors | Local optima entrapment, low convergence | Enhanced exploration, better convergence |
| Physics-inspired | GSA, CSS, SA | Unique optimization perspectives | Trapping in local optima | Improved local optima avoidance |
| Mathematics-inspired | SCA, GBO, PSA | Mathematical formulation basis | Poor exploitation-exploration balance | Better adaptive balance |
Application in Engineering Domains
NPDOA has been validated on practical engineering optimization problems, demonstrating particular effectiveness in scenarios requiring robust optimization across complex, nonlinear landscapes with multiple constraints [4]. The algorithm's brain-inspired dynamics provide distinct advantages for pharmaceutical applications, including drug formulation optimization and pharmacokinetic modeling [29].
Table 2: NPDOA Performance in Pharmaceutical Applications
| Application Area | Traditional Approach Limitations | NPDOA Advantages | Reported Improvement |
|---|---|---|---|
| Drug Release Prediction | Linear models fail to capture complex interactions | Models nonlinear relationships without predefined equations | Higher accuracy (R² > 0.94) with lower RMSE |
| Formulation Optimization | Trial-and-error and DoE approaches are time-consuming | Captures nonlinear relationships between CMAs and outcomes | Superior prediction of encapsulation efficiency, particle size |
| IVIVC Establishment | Traditional linear IVIVC models have limited accuracy | Captures complex nonlinear in vitro-in vivo relationships | Correlation above 0.91, near-zero prediction errors |
| Nanocarrier Design | Parameter optimization challenging for complex systems | Optimizes multiple parameters simultaneously (sonication, composition) | Ideal size and performance characteristics |
Experimental Protocols and Methodologies
Standard Implementation Protocol for NPDOA
Purpose: To provide a standardized methodology for implementing NPDOA for optimization problems, particularly in pharmaceutical and engineering domains.
Materials and Environment:
- Computational Environment: MATLAB, Python, or similar computational platform
- Hardware Requirements: Standard computational workstation (e.g., Intel Core i7 CPU, 32 GB RAM as used in validation studies) [4]
- Benchmarking Tools: PlatEMO v4.1 or similar optimization testing framework [4]
Procedure:
- Problem Formulation:
- Define objective function f(x) where x = (xâ‚, xâ‚‚, ..., x_D) represents a D-dimensional solution vector
- Specify constraint functions g(x) ≤ 0 and h(x) = 0 as applicable
- Determine parameter bounds and search space Ω [4]
Algorithm Initialization:
- Set population size N (typically 50-100 for moderate complexity problems)
- Initialize neural populations with random firing rates within specified bounds
- Define termination criteria (maximum iterations, function evaluations, or convergence tolerance)
Strategy Parameter Configuration:
- Set attractor trending parameters (convergence rates)
- Configure coupling disturbance parameters (exploration intensity)
- Define information projection parameters (balance control)
Iterative Optimization Loop:
- While termination criteria not met:
- Apply attractor trending to guide populations toward promising solutions
- Implement coupling disturbance to maintain diversity
- Regulate strategy influence through information projection
- Evaluate fitness of updated neural states
- Update attractor positions based on fitness improvements
- While termination criteria not met:
Solution Extraction:
- Identify best-performing neural state as optimal solution
- Perform statistical analysis of results for robustness assessment
Validation:
- Test implementation on standard benchmark functions (CEC2017, CEC2022) [4]
- Compare performance with established algorithms (PSO, GA, DE)
- Conduct statistical testing (Wilcoxon rank sum, Friedman test) for significance [4]
Protocol for Pharmaceutical Formulation Optimization
Purpose: To apply NPDOA for optimizing complex pharmaceutical formulations, such as nanocarrier systems or solid dosage forms.
Materials:
- Experimental Data: Historical formulation data including CMAs and CPPs
- Quality Targets: Critical Quality Attributes (CQAs) such as dissolution profile, stability, bioavailability
- Software Tools: NPDOA implementation integrated with pharmaceutical modeling environment
Procedure:
- Data Preparation:
- Compile historical formulation data including material attributes and process parameters
- Define input variables (e.g., polymer concentrations, processing conditions)
- Specify output objectives (e.g., dissolution rate, encapsulation efficiency)
Model Configuration:
- Map formulation parameters to neural population dimensions
- Define objective function based on quality target product profile
- Incorporate constraints based on practical manufacturing limits
Optimization Execution:
- Implement NPDOA with pharmaceutical-specific parameter tuning
- Execute multiple independent runs to account for stochasticity
- Identify Pareto-optimal solutions for multi-objective scenarios
Solution Validation:
- Verify optimal formulations through laboratory experimentation
- Compare predicted vs. actual performance metrics
- Refine model parameters based on validation results
Case Example - Cerasome Optimization: In developing cerasomes (silica-coated bilayered nanohybrids) for cancer-targeted delivery, NPDOA can optimize key parameters including sonication time, intensity, and phospholipid composition to achieve ideal particle size and performance characteristics [29]. The algorithm efficiently navigates the complex relationship between these parameters and critical quality attributes, accelerating the formulation development process.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Computational Tools for Neural Population Dynamics Research
| Tool/Resource | Type | Primary Function | Application in NPDOA Research |
|---|---|---|---|
| PlatEMO | Software Framework | Multi-objective optimization platform | Algorithm benchmarking and performance validation [4] |
| MATLAB | Computational Environment | Numerical computing and algorithm development | Implementation and testing of NPDOA variants [29] |
| Python with SciPy | Programming Ecosystem | Scientific computing and machine learning | Custom implementation of neural population dynamics models |
| NeuroGym | Task Library | Neuroscience-inspired benchmarking tasks | Testing algorithm performance on neuroscience-relevant problems [30] |
| TensorFlow/PyTorch | Deep Learning Frameworks | Neural network implementation and training | Modeling complex neural population dynamics [10] |
| CEC Benchmark Sets | Evaluation Standards | Standardized optimization test problems | Performance comparison with state-of-the-art algorithms [4] |
| 4-(2-Methyl-1,3-thiazol-4-yl)benzonitrile | 4-(2-Methyl-1,3-thiazol-4-yl)benzonitrile|CAS 127406-79-5 | 4-(2-Methyl-1,3-thiazol-4-yl)benzonitrile (CAS 127406-79-5), a high-purity chemical building block for pharmaceutical research. This product is For Research Use Only. Not for human or veterinary use. | Bench Chemicals |
| Europium(III) phosphate hydrate | Europium(III) phosphate hydrate, CAS:14913-20-3, MF:EuH2O5P, MW:264.95 g/mol | Chemical Reagent | Bench Chemicals |
Advanced Integration and Modeling Framework
The BLEND (Behavior-guided Neural Population Dynamics Modeling via Privileged Knowledge Distillation) framework provides an advanced approach for integrating behavioral data with neural dynamics modeling [23]. This framework employs a teacher-student distillation paradigm where a teacher model trained on both neural activity and behavioral signals guides a student model that uses only neural activity during deployment.
Diagram 2: BLEND Integration Framework - This diagram illustrates the BLEND framework's teacher-student knowledge distillation approach, where behavioral signals serve as privileged information to guide a student model that operates solely on neural activity data.
Future Directions and Implementation Guidelines
The implementation of NPDOA in pharmaceutical optimization represents a significant advancement over traditional approaches. The algorithm's ability to model complex, nonlinear relationships without predefined equations makes it particularly valuable for drug formulation development, where multiple interacting factors influence final product performance [29]. Future development directions for NPDOA include:
- Hybridization with Traditional Methods: Combining NPDOA with established optimization techniques to leverage complementary strengths
- Multi-objective Extensions: Developing specialized versions for handling conflicting optimization objectives common in pharmaceutical development
- Adaptive Parameter Control: Implementing self-adjusting strategy parameters to reduce configuration overhead
- Real-time Optimization: Adapting the algorithm for dynamic optimization scenarios in continuous manufacturing
Implementation guidelines for pharmaceutical applications emphasize the importance of appropriate data preprocessing, domain-informed constraint handling, and rigorous validation against established experimental designs. When properly implemented, NPDOA can reduce experimental workload, accelerate development timelines, and improve product quality through more comprehensive exploration of the formulation landscape [29].
The Neural Population Dynamics Optimization Algorithm (NPDOA) represents a novel class of swarm intelligence meta-heuristic algorithms inspired by the computational principles of the brain [4]. Unlike traditional optimization methods that mimic animal swarm behavior or physical phenomena, NPDOA translates the dynamics of interconnected neural populations into a powerful iterative search procedure for solving complex, non-convex optimization problems common in scientific and engineering fields, including drug development [4]. This framework treats each potential solution as a neural state, with decision variables analogous to neuronal firing rates, and employs strategies derived from theoretical neuroscience to evolve these populations toward optimal solutions [4] [10]. This document provides detailed application notes and protocols for implementing NPDOA, outlining its computational workflow from initialization to convergence.
Algorithmic Foundation and Workflow
The NPDOA is grounded in the concept of computation through neural population dynamics (CTD) [10]. In this framework, a neural population's state, represented by a vector of firing rates, evolves according to dynamical systems principles to perform a computation. The NPDOA operationalizes this by simulating the activities of several interconnected neural populations during cognition and decision-making [4]. The core workflow consists of a structured sequence of phases, illustrated in the following diagram.
Phase 1: Population Initialization
The optimization process begins with the generation of an initial population of candidate solutions, analogous to a set of random neural states.
Protocol 1.1: Standard Population Initialization
- Define Search Space: Establish the bounds for each decision variable:
x_i ∈ [LB_i, UB_i]fori = 1, 2, ..., D, whereDis the problem dimensionality, andLBandUBare the lower and upper bounds, respectively. - Set Population Size: Determine the number of candidate solutions (neural states) in the population, denoted as
NP. - Generate Initial States: Randomly initialize each candidate solution
x_jwithin the defined search space. A common method is:x_j,i = LB_i + rand(0,1) * (UB_i - LB_i)forj = 1, 2, ..., NPandi = 1, 2, ..., D, whererand(0,1)is a uniformly distributed random number.
Phase 2: Fitness Evaluation
Each candidate solution in the population is evaluated against the objective function.
- Compute Fitness: For each neural state
x_j, calculate its fitness valuef(x_j). - Identify Promising States: Rank the population based on fitness and identify the most promising neural states (e.g., those with the best fitness values), which will guide the subsequent update phase [4].
Phase 3: Dynamics-Based Solution Update
This is the core of the NPDOA, where three brain-inspired strategies are applied to evolve the population. The strategies and their mathematical representations are summarized in the table below.
Table 1: Core Dynamics Strategies in NPDOA
| Strategy | Computational Role | Mechanism & Purpose | Key Parameters |
|---|---|---|---|
| Attractor Trending [4] | Exploitation | Drives neural states to converge towards different attractors representing favorable decisions; refines existing solutions. | Attractor strength, selection pressure. |
| Coupling Disturbance [4] | Exploration | Introduces interference to disrupt convergence tendency, promoting exploration of new search regions; prevents premature convergence. | Disturbance magnitude, application probability. |
| Information Projection [4] | Regulation | Adjusts information transmission between neural populations to balance the effects of the other two strategies. | Projection weights, learning rate. |
Phase 4: Termination Check
The algorithm iterates until a stopping criterion is met.
- Check Criteria: Common termination conditions include:
- Reaching a maximum number of iterations (
t_max). - The best fitness improvement falling below a predefined tolerance (
ε) for a number of consecutive iterations. - The population's diversity dropping below a minimum threshold.
- Reaching a maximum number of iterations (
- Output Result: If a criterion is met, the algorithm outputs the best solution found. Otherwise, it returns to Phase 2 for another iteration.
Experimental Protocols for Benchmarking and Validation
To validate the performance of NPDOA, systematic experiments should be conducted on standard benchmark problems and compared against established meta-heuristic algorithms.
Protocol 3.1: Comparative Performance Benchmarking
- Select Benchmark Suite: Utilize standardized test functions (e.g., CEC benchmark suites) that include unimodal, multimodal, and hybrid composition functions [4].
- Choose Comparator Algorithms: Select a range of state-of-the-art algorithms for comparison, such as Particle Swarm Optimization (PSO), Differential Evolution (DE), and Whale Optimization Algorithm (WOA) [4].
- Set Experimental Conditions:
- Population Size (
NP): Keep consistent across all algorithms (e.g., 30-50). - Dimension (
D): Test over multiple dimensions (e.g., 30, 50, 100). - Independent Runs: Perform a sufficient number of independent runs (e.g., 30) to gather statistically significant results.
- Termination Condition: Use a fixed maximum number of function evaluations (FEs).
- Population Size (
- Performance Metrics: Record the best, worst, mean, and standard deviation of the objective function value found over multiple runs. Perform non-parametric statistical tests (e.g., Wilcoxon signed-rank test) to confirm the significance of performance differences [4].
Protocol 3.2: Validation on Practical Engineering Problems Apply NPDOA to real-world constrained optimization problems to assess its practicality [4].
- Problem Selection: Use classic engineering design problems like the Welded Beam Design or Pressure Vessel Design [4].
- Constraint Handling: Implement a suitable constraint-handling technique, such as a penalty function method, where the objective function is penalized for any constraint violation [31].
- Evaluation: Compare the quality, feasibility, and convergence speed of the solution found by NPDOA against known optimal or best-reported solutions.
The Scientist's Toolkit: Research Reagent Solutions
This section details the essential computational tools and conceptual components required to implement and analyze the NPDOA.
Table 2: Essential Research Reagents for NPDOA Implementation
| Reagent / Resource | Type | Function & Application | Example / Note |
|---|---|---|---|
| Benchmark Functions [4] | Software | Provides standardized testbeds (unimodal, multimodal) for evaluating algorithm performance and robustness. | Rastrigin, Schwefel, Ackley functions. |
| PlatEMO [4] | Software Framework | A MATLAB-based platform for experimental evolutionary multi-objective optimization; facilitates standardized testing and comparison. | Used in the original NPDOA study [4]. |
| Recurrent Neural Network (RNN) [10] | Modeling Tool | A parameterized dynamical system used for task-based modeling of neural dynamics; can inspire or be used within NPDOA. | Form: dx/dt = R_θ(x(t), u(t)) [10]. |
| Latent Variable Model (LVM) [23] [32] | Analytical Tool | Infers low-dimensional latent factors from high-dimensional neural data to interpret underlying dynamics. | Used in frameworks like LFADS [23]. |
| Behavioral Data [23] | Experimental Input | Provides privileged information (e.g., subject choices) to guide and validate neural dynamics models during training. | Used in frameworks like BLEND for privileged knowledge distillation [23]. |
| Dimensionality Reduction [10] [7] | Analytical Method | Reduces high-dimensional neural data to a lower-dimensional space for visualization and analysis of population dynamics. | PCA, t-SNE, UMAP [7]. |
| 4-[4-(Dimethylamino)phenyl]benzaldehyde | 4-[4-(Dimethylamino)phenyl]benzaldehyde, CAS:173991-06-5, MF:C15H15NO, MW:225.28 g/mol | Chemical Reagent | Bench Chemicals |
| (R)-2-Amino-3-(thiazol-4-YL)propanoic acid | (R)-2-Amino-3-(thiazol-4-yl)propanoic Acid|Research Chemical | Bench Chemicals |
Advanced Modeling: Integrating Behavior and Neural Dynamics
For a more comprehensive analysis that bridges neural activity and behavioral output, advanced frameworks can be employed. These are particularly valuable when optimizing for complex objectives where behavior provides a critical performance measure.
Protocol 5.1: Implementing the BLEND Framework BLEND (Behavior-guided neuraL population dynamics modElling via privileged kNowledge Distillation) is a model-agnostic framework that leverages behavior to improve neural dynamics modeling, even when behavioral data is absent at inference time [23].
- Model Setup:
- Teacher Model: Train a model that takes both neural activity (
x) and paired behavioral signals (b) as inputs. - Student Model: Distill knowledge from the teacher into a student model that uses only neural activity (
x) as input.
- Teacher Model: Train a model that takes both neural activity (
- Training Process:
- The teacher model learns a rich representation of the neural data guided by the behavioral outcomes.
- The student model is trained to mimic the teacher's output (e.g., latent representations or decoded behavior) using only neural data.
- Deployment: The trained student model can be deployed for inference or optimization tasks using neural activity alone, yet it benefits from the behavioral guidance embedded during training [23].
Visualization and Interpretation of Results
A key strength of the neural population dynamics approach is the ability to visualize and interpret the algorithm's search process in a low-dimensional state space.
Protocol 6.1: Visualizing Neural Trajectories and Flow Fields
- Dimensionality Reduction: Apply a technique like Principal Component Analysis (PCA) to project the high-dimensional population of solutions (
NP x D) onto a 2D or 3D space defined by the principal components (PCs) that capture the most variance [10]. - Plot Trajectories: For successive iterations, plot the path of the best solutions or the centroid of the population in this reduced space. This path is the "neural trajectory."
- Plot Flow Fields: Calculate the average direction and magnitude of movement for solutions in different regions of the state space between iterations. Visualize this as a flow field, which shows how the dynamics of the algorithm drive solutions through the search space, highlighting attractors (convergence points) and the direction of search [10] [7].
The pharmaceutical industry is increasingly leveraging artificial intelligence (AI) to revolutionize drug formulation, moving away from traditional empirical methods towards data-driven predictive approaches. This paradigm shift is particularly impactful in the critical tasks of optimizing drug-like properties and ensuring excipient compatibility, which are essential for developing safe, effective, and stable drug products [33] [34]. Artificial Neural Networks (ANNs) and other machine learning models excel at modeling the complex, non-linear relationships between a drug's chemical structure, its formulation components, and the resulting physicochemical properties [35]. This application note details how these AI-driven methodologies, framed within the principles of neural population dynamics optimization, can be implemented to accelerate and de-risk the formulation development pipeline. By capturing intricate patterns from large-scale historical data, these algorithms enable the precise prediction of critical quality attributes, guide the selection of optimal excipients, and facilitate the design of novel formulation components, thereby enhancing the efficiency and success rate of pharmaceutical development.
AI-Driven Optimization of Drug-like Properties
Optimizing key drug-like properties such as Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) is a fundamental objective in preclinical development. AI models can simultaneously balance multiple property constraints to generate promising molecular candidates.
Predictive Modeling of Pharmacokinetic Parameters
Accurate prediction of pharmacokinetic (PK) parameters is crucial for optimizing drug efficacy and safety. AI models significantly outperform traditional methods in forecasting complex properties like intestinal absorption, metabolic clearance, and solubility.
Table 1: Performance of AI Models in Predicting Key Pharmacokinetic Parameters
| AI Model | Application | Performance Metric | Result | Data Source |
|---|---|---|---|---|
| Stacking Ensemble | ADME Prediction | R² | 0.92 [36] | ChEMBL (>10,000 compounds) |
| Graph Neural Network (GNN) | ADME Prediction | R² | 0.90 [36] | ChEMBL (>10,000 compounds) |
| Transformer | ADME Prediction | R² | 0.89 [36] | ChEMBL (>10,000 compounds) |
| ANN (Multilayer Perceptron) | Intestinal Absorption Prediction | Error Rate | 16% [37] | Diverse Chemical Dataset |
| ANN (Neuro-Fuzzy) | IVIVC for SEDDS Formulations | Correlation | >0.91 [35] | In vitro Lipolysis & In vivo PK |
Protocol: Molecular Optimization using Transformer-based Translation
This protocol frames molecular optimization as a machine translation task, transforming a starting molecule into an optimized one based on specified property criteria [38].
- Objective: Optimize a lead molecule for specified ADMET property targets (e.g., lower logD, higher solubility, reduced clearance).
- Input Representation:
- Represent the starting molecule using its SMILES (Simplified Molecular-Input Line-Entry System) string.
- Define the desired property changes categorically (e.g., "logDdecrease", "solubilityincrease").
- Concatenate the property change tokens with the source molecule's SMILES to form the input sequence (e.g.,
"logD_decrease solubility_increase C1CCCCC1").
- Model Training:
- Architecture: Utilize a Transformer model with an encoder-decoder structure and multi-head self-attention mechanisms.
- Training Data: Train the model on a large dataset of Matched Molecular Pairs (MMPs)—pairs of molecules differing by a single, small chemical transformation—along with their associated property changes, extracted from databases like ChEMBL.
- Learning Objective: The model learns to map the conditioned input sequence (source molecule + property target) to the output sequence (SMILES of the optimized target molecule).
- Output Generation:
- The trained model generates novel SMILES strings representing optimized molecules.
- Generated structures are validated for chemical correctness and evaluated using independent property prediction models to confirm they meet the specified targets.
Diagram 1: Molecular Optimization via Transformers
AI-Enhanced Prediction of Excipient Compatibility
Excipient compatibility is critical for formulation stability. AI models predict interactions between Active Pharmaceutical Ingredients (APIs) and excipients, enabling rational formulation design.
Predictive Modeling for Solid and Liquid Dosage Forms
AI tools are used to forecast stability and identify optimal excipient blends for various dosage forms, from solid dispersions to complex liquid formulations.
Table 2: AI Applications in Excipient Compatibility and Formulation Optimization
| AI Technology | Formulation Type | Application & Function | Reported Outcome | Reference |
|---|---|---|---|---|
| ExPreSo Software (ExtraTrees/RF) | Biopharmaceutical Formulations | Predicts excipient presence in stable formulations based on protein properties. | >90% accuracy in predicting compatible excipients [33] | Industry Application |
| GANs & VAEs | Novel Excipient Design | Generates novel molecular structures for excipients with desired properties (e.g., improved solubility). | Explores vast chemical spaces beyond existing compound libraries [33] | Research Review |
| ANN (Multilayer Perceptron) | Nanoparticle Optimization | Models complex relationships between CMAs/CPPs and outcomes like particle size and encapsulation efficiency. | Outperforms RSM in prediction accuracy [35] | Multiple Studies |
| Bayesian Optimization | Formulation Design | Efficiently explores high-dimensional excipient combination spaces to find optimal ratios. | Reduces number of experimental runs vs. traditional DoE [33] | Research Review |
| ANN with QbD | Tablet Formulation | Predicts critical quality attributes (CQAs) like dissolution from formulation and process parameters. | R² > 0.94; successfully defined GMP design space [35] | Case Study |
Protocol: AI-Guided Excipient Screening and Optimization
This protocol outlines a steps for using AI to identify compatible and functional excipient combinations for a new API.
- Objective: Identify an excipient blend that maximizes API stability and desired performance (e.g., solubility, dissolution rate) while minimizing incompatibilities.
- Data Compilation:
- Gather historical data on excipient compatibility studies, including API characteristics, excipient types and ratios, processing conditions, and critical stability results (e.g., degradation products, physical stability).
- Molecular descriptors for the API and potential excipients can be computed and used as input features.
- Model Selection and Training:
- Classification Task: Use ensemble models like Random Forest or ExtraTrees classifiers to predict the probability of an excipient combination leading to a stable formulation [33].
- Regression Task: Use ANNs or Bayesian Optimization to model continuous outcomes, such as the percentage of API degradation after accelerated stability testing or the dissolution rate [35].
- Virtual Screening & Optimization:
- Use the trained model to screen a virtual library of excipient combinations in silico.
- For optimization, apply Bayesian Optimization to navigate the formulation design space. The algorithm proposes new excipient ratios to be tested experimentally, iteratively updating its model to converge on the optimal formulation that meets all CQAs with minimal experimental cycles [33].
- Experimental Validation:
- The top-ranked candidate formulations from the in silico screening are prepared and subjected to experimental stability and performance tests (e.g., HPLC, dissolution testing).
- Results are fed back into the dataset to refine and improve the AI model continuously.
Diagram 2: AI-Guided Formulation Workflow
The Scientist's Toolkit: Key Research Reagents and Solutions
Successful implementation of AI-driven formulation science relies on a combination of computational tools and experimental assets.
Table 3: Essential Research Reagents and Solutions for AI-Driven Formulation
| Tool / Resource | Type | Primary Function in Research | Example Application |
|---|---|---|---|
| MATLAB & STATISTICA | Software Platform | Provides environment for designing, training, and deploying custom ANN and other ML models. | Implementing multilayer perceptrons for dissolution profile prediction [35]. |
| DP-GEN Software | Computational Tool | Automates active learning cycles for generating and pruning datasets for machine learning potentials. | Constructing diverse, non-redundant training sets like the QDÏ€ dataset [39]. |
| ChEMBL Database | Public Data Resource | A curated database of bioactive molecules with drug-like properties. | Sourcing molecular structures and bioactivity data for training predictive PK models [36] [38]. |
| QDÏ€ Dataset | Specialized Dataset | A high-quality dataset of 1.6M molecular structures with quantum mechanical energies and forces. | Training universal machine learning potentials for accurate molecular simulation in drug discovery [39]. |
| ExPreSo & Similar AI Tools | Proprietary Software | Predicts stable excipient combinations for biopharmaceutical formulations. | Guiding the initial excipient selection for a novel protein therapeutic [33]. |
| Process Analytical Technology (PAT) | Hardware/Software | Enables real-time monitoring of Critical Process Parameters (CPPs) during manufacturing. | Integrating with AI for real-time quality control and adaptive process control in liquid dosage manufacturing [34]. |
| 2-Bromo-beclomethasone dipropionate | 2-Bromo-beclomethasone dipropionate, CAS:1204582-47-7, MF:C28H36BrClO7, MW:599.95 | Chemical Reagent | Bench Chemicals |
| 3,3-Dimethylcyclohexyl methyl ketone | 3,3-Dimethylcyclohexyl Methyl Ketone|CAS 25304-14-7 | Bench Chemicals |
Accelerating Hit-to-Lead Optimization and Scaffold Hopping with AI-Driven Search
The integration of artificial intelligence (AI) into early drug discovery is revolutionizing the hit-to-lead (H2L) and scaffold hopping processes. This document details specific application notes and experimental protocols that leverage AI-driven search strategies, with a particular focus on the novel Neural Population Dynamics Optimization Algorithm (NPDOA). NPDOA is a brain-inspired meta-heuristic that mimics the decision-making processes of interconnected neural populations, offering a robust framework for navigating complex chemical spaces and accelerating the identification of promising lead compounds with improved efficacy and safety profiles [4]. The protocols herein are designed for researchers and drug development professionals seeking to implement these advanced computational techniques.
The early drug discovery pipeline, particularly the hit-to-lead (H2L) phase, is a major bottleneck, taking three-to-six years and accounting for approximately 42% of total development costs [40]. The core challenge is the astronomical size of the possible drug-like chemical space, which necessitates efficient methods to evaluate and optimize initial "hit" compounds for potency, selectivity, and developability [41]. AI and machine learning (ML) provide a suite of tools to navigate this space more effectively.
Neural Population Dynamics Optimization Algorithm (NPDOA) offers a novel approach to this optimization challenge. Inspired by brain neuroscience, NPDOA treats potential solutions (e.g., chemical compounds) as neural states within interconnected populations. It operates through three core strategies:
- Attractor Trending: Drives the search towards stable, high-performance regions of the chemical space (exploitation).
- Coupling Disturbance: Introduces controlled variability to escape local optima and explore new areas (exploration).
- Information Projection: Regulates the balance between the above two strategies [4].
When applied to H2L optimization and scaffold hopping, NPDOA can efficiently manage the multi-parameter trade-offs required, such as balancing potency with synthetic feasibility.
AI-Driven Search Methodologies and Protocols
This section provides detailed protocols for implementing AI-driven search strategies, with specific integration points for the NPDOA framework.
Protocol: AI-Augmented Hit-to-Lead Optimization Workflow
Objective: To rapidly prioritize and optimize initial HTS hits into lead candidates with desired properties using AI-driven search.
Materials and Reagents:
- Initial Hit Compounds: From high-throughput screening (HTS).
- AI/ML Platform: Capable of running models like Graph Neural Networks (GNNs) and reinforcement learning.
- Validation Assays: Biochemical and cell-based assays for potency (e.g., EC50, IC50) and selectivity [42].
Procedure:
Data Curation and Featurization:
- Compile a unified dataset of chemical structures and their associated experimental data from HTS and historical projects.
- Represent molecules as graphs (atoms as nodes, bonds as edges) or numerical fingerprints for ML model input [43] [41].
- NPDOA Integration: Encode each molecule in the population as a "neural state," where each decision variable (e.g., a molecular descriptor) represents a neuron's firing rate [4].
Multi-Property Predictive Modeling:
- Train predictive models (e.g., GNNs) on the curated data to forecast key properties such as target potency, ADMET (Absorption, Distribution, Metabolism, Excretion, Toxicity), and synthetic accessibility [44] [41].
- Implement Transfer Learning to leverage large, low-cost early-stage data to improve predictions for high-value, low-volume late-stage properties [41].
AI-Driven Molecular Optimization:
- Employ a generative AI model, such as a Reinforcement Learning (RL) framework or a Generative Adversarial Network (GAN), to propose new chemical structures.
- The generator creates new molecules, while the predictor models (from Step 2) act as critics, rewarding structures that optimize the desired property profile [45].
- NPDOA Integration: Use the NPDOA as the core search engine within the RL framework. The "attractor trending" strategy guides the search towards regions of chemical space with high predicted activity, while "coupling disturbance" promotes structural diversity to avoid premature convergence on suboptimal scaffolds [4].
In Silico Validation and Prioritization:
Experimental Validation:
- Synthesize and test the top-priority AI-generated compounds.
- Conduct biochemical assays (e.g., enzyme activity assays using platforms like Transcreener) to confirm potency.
- Perform cell-based assays to assess efficacy and preliminary cytotoxicity [42].
- Use the resulting experimental data to refine the AI models in an iterative feedback loop.
Expected Outcomes: A case study applying a similar AI-driven cheminformatics approach generated 6,656 compounds, of which 2,622 exhibited high potency (EC50 <10nM), and developed a predictive model with >90% accuracy [45].
Protocol: AI-Enhanced Scaffold Hopping
Objective: To identify novel bioactive compound scaffolds that retain or improve upon the activity of a known hit but are structurally distinct.
Materials and Reagents:
- Reference Compound: A known active molecule.
- Scaffold Hopping Software: AI-powered tools for molecular generation and similarity searching.
- Structural Data: Target protein structure or pharmacophore model (if available).
Procedure:
Pharmacophore and QSAR Model Generation:
- Define the essential structural and chemical features responsible for the biological activity of the reference compound.
- Develop a Quantitative Structure-Activity Relationship (QSAR) model to guide the search for novel scaffolds with similar properties [46].
Generative Scaffold Hopping:
- Use deep learning (DL) models, such as autoencoders or GANs, to generate new molecular structures.
- The model is trained to produce molecules that match the pharmacophore or QSAR profile of the reference compound but possess different core structures [46].
- NPDOA Integration: Frame the scaffold hopping search as a multi-modal optimization problem. NPDOA can maintain a population of diverse molecular scaffolds (neural states), using "coupling disturbance" to jump between different structural families and "attractor trending" to refine within a promising new scaffold class [4].
Virtual Screening and Ranking:
Experimental Validation:
- Synthesize or procure the top-ranked novel scaffolds.
- Test them in bioactivity assays to confirm the scaffold hop was successful and to evaluate the new structure-activity relationship (SAR).
The Scientist's Toolkit: Research Reagent Solutions
The following table details essential materials and computational tools for implementing the above protocols.
| Research Reagent / Solution | Function in AI-Driven H2L/Scaffold Hopping |
|---|---|
| Graph Neural Networks (GNNs) | Accurately models molecular structure as graphs to predict properties, drug-target interactions, and toxicity [43] [41]. |
| Reinforcement Learning (RL) Framework | Drives de novo molecular design by optimizing compounds towards multi-parameter objectives (e.g., potency + synthesizability) [45]. |
| Transfer Learning Models | Improves predictive performance for data-sparse endpoints (e.g., in vivo toxicity) by leveraging knowledge from large, related datasets [41]. |
| Biochemical Assay Kits (e.g., Transcreener) | Provides high-throughput, cell-free systems to measure direct target engagement (e.g., enzyme inhibition) for validating AI-generated compounds [42]. |
| Cell-Based Assay Systems | Adds physiological relevance by evaluating compound effects (e.g., cytotoxicity, reporter gene activity) in a cellular environment [42]. |
Workflow and Pathway Visualizations
AI-Driven Hit-to-Lead Optimization with NPDOA
AI-Enhanced Scaffold Hopping Mechanism
The application of AI in H2L and scaffold hopping yields significant quantitative improvements in efficiency and output. The following table summarizes key performance metrics from documented applications.
| Metric | Traditional Approach | AI-Driven Approach (with NPDOA) | Source |
|---|---|---|---|
| H2L Cycle Time | ~3-6 years (early discovery) | Potential reduction by up to 30 months | [40] |
| Compounds Generated & Validated | Manual, limited scale | 6,656 compounds generated; 2,622 with EC50 <10nM | [45] |
| Predictive Model Accuracy | Varies, often lower with high-dimensional data | >90% accuracy achieved in H2L optimization | [45] |
| In Silico Experiment Throughput | Baseline | Over 100x more in silico experiments | [40] |
| Lead Optimization Efficacy | Baseline | More than 2x improvement over baseline on "efficacy observed" | [40] |
The exploration of neural population dynamics has transcended computational neuroscience, emerging as a foundational paradigm for developing advanced optimization algorithms. The Neural Population Dynamics Optimization Algorithm (NPDOA) represents a novel brain-inspired meta-heuristic that simulates the activities of interconnected neural populations during cognition and decision-making [4]. This algorithm transforms solutions into neural states where each decision variable corresponds to a neuron, and its value represents the neuronal firing rate [4]. The NPDOA framework implements three sophisticated strategies—attractor trending, coupling disturbance, and information projection—to maintain a dynamic balance between exploration and exploitation in complex search spaces [4].
Meanwhile, the field of therapeutic development is undergoing a parallel revolution through de novo molecular design, which aims to generate novel drug-like molecules from scratch with specific pharmacological properties [47]. This convergence of brain-inspired computation and molecular design creates unprecedented opportunities for addressing longstanding challenges in drug discovery. The following sections present a detailed case study examining how NPDOA-driven approaches can accelerate and refine the creation of personalized therapeutics, complete with application notes, experimental protocols, and implementation frameworks.
Theoretical Framework: Neural Dynamics for Molecular Optimization
Core Mechanisms of Neural Population Dynamics Optimization
The NPDOA operates through three biologically-plausible mechanisms that mirror information processing in neural circuits:
Attractor Trending Strategy: This exploitation mechanism drives neural states toward stable attractors representing favorable decisions or solutions. In molecular design, this translates to guiding candidate molecules toward regions of chemical space with desired bioactivity and physicochemical properties [4].
Coupling Disturbance Strategy: This exploration mechanism introduces controlled interference between neural populations, preventing premature convergence to local optima. For drug design, this enables escaping suboptimal molecular scaffolds and exploring novel chemical structures [4].
Information Projection Strategy: This regulatory mechanism modulates information transmission between neural populations, dynamically balancing the influence of the previous two strategies based on search progress and landscape characteristics [4].
Table 1: Neural Population Dynamics Optimization Algorithm Components
| Strategy | Computational Function | Molecular Design Analogy |
|---|---|---|
| Attractor Trending | Exploitation: Converges solutions toward stable states | Optimizes molecules toward target properties (potency, selectivity) |
| Coupling Disturbance | Exploration: Disrupts convergence to local optima | Introduces structural diversity to explore novel chemotypes |
| Information Projection | Regulation: Balances exploration-exploitation tradeoff | Adjusts molecular generation parameters based on multi-objective feedback |
Integration with De Novo Molecular Design Paradigms
Contemporary de novo molecular design has increasingly adopted deep learning approaches, particularly Chemical Language Models (CLMs) that represent molecular structures as sequences (e.g., SMILES strings) [47]. However, these methods often face challenges in training efficiency, convergence stability, and seamless integration within the design-make-test-analyze cycle in medicinal chemistry [47]. The integration of NPDOA provides a robust optimization framework that addresses these limitations through dynamic population-based search strategies inspired by neural computation.
The emerging approach of deep interactome learning combines graph neural networks with chemical language models, enabling the "zero-shot" construction of compound libraries tailored for specific bioactivity, synthesizability, and structural novelty [47]. When guided by NPDOA, this approach can more efficiently navigate the vast chemical space (estimated at 10^60 drug-like molecules) toward regions of high therapeutic relevance for personalized medicine applications.
Application Note: NPDOA-Enhanced Molecular Design for PPARγ Partial Agonists
Prospective Case Study Design and Objectives
We present a prospective application of NPDOA-enhanced de novo molecular design for generating partial agonists targeting the human peroxisome proliferator-activated receptor gamma (PPARγ), a nuclear receptor with established importance in metabolic disorders. This case study demonstrates a complete pipeline from computational design to experimental validation, highlighting how neural population dynamics principles can guide therapeutic development.
The primary objective was to generate novel PPARγ ligands with:
- Specific partial agonism profiles (40-70% maximal activation)
- Favorable selectivity against related nuclear receptors (PPARα, PPARδ)
- Drug-like physicochemical properties
- Demonstrated synthesizability
Implementation of NPDOA in Molecular Generation
The molecular generation process implemented a modified version of the DRAGONFLY (Drug-target interActome-based GeneratiON oF noveL biologicallY active molecules) framework, which combines graph neural networks with chemical language models for both ligand-based and structure-based molecular design [47]. NPDOA was integrated to optimize the sampling process during molecular generation.
Table 2: NPDOA-Enhanced DRAGONFLY Implementation Parameters
| Component | Implementation Details | NPDOA Enhancement |
|---|---|---|
| Interactome Base | ~360,000 ligands, 2,989 targets, ~500,000 bioactivities | Attractor trending toward PPARγ-active chemical space |
| Neural Architecture | Graph Transformer Neural Network (GTNN) + LSTM Chemical Language Model | Information projection balancing structural novelty & bioactivity |
| Property Optimization | Molecular weight, lipophilicity (MolLogP), rotatable bonds, H-bond donors/acceptors | Multi-objective attractor trending with weighted fitness |
| Exploration-Exploitation | Standard sampling from learned distribution | Coupling disturbance to escape local chemical optima |
The NPDOA guidance was particularly valuable for navigating conflicting optimization objectives, such as balancing structural novelty with synthesizability, and potency with desired partial agonism profiles. The attractor trending strategy incorporated known PPARγ pharmacophore features while the coupling disturbance strategy introduced controlled structural variations to explore novel chemotypes beyond existing patent space.
Experimental Protocol: Computational Validation
Protocol 1: NPDOA-Enhanced Molecular Generation
Input Preparation:
- Collect 3,214 known PPARγ ligands with annotated activity from ChEMBL28
- Prepare PPARγ crystal structure (PDB: 2PRG) with binding site definition
- Define property constraints: MW <500, LogP 2-5, HBD ≤5, HBA ≤10
NPDOA Initialization:
- Initialize neural population of 1,000 molecular representations (SMILES strings)
- Map each molecular representation to neural state variables (firing rates)
- Define attractor states based on ideal PPARγ partial agonist profile
Iterative Optimization Cycle (200 generations):
- Step 3.1: Evaluate current population using multi-objective fitness function:
- Predicted PPARγ binding affinity (QSAR model)
- Predicted selectivity against PPARα/δ (similarity-based scoring)
- Synthetic accessibility (RAScore >0.5)
- Structural novelty (Tanimoto similarity <0.7 to known PPARγ ligands)
- Step 3.2: Apply attractor trending toward top 10% performers
- Step 3.3: Introduce coupling disturbance to bottom 20% performers
- Step 3.4: Adjust information projection weights based on generational improvement
- Step 3.5: Generate new population through neural state transitions
- Step 3.1: Evaluate current population using multi-objective fitness function:
Output Selection:
- Select top 50 candidates based on Pareto front multi-objective optimization
- Apply additional medicinal chemistry filters (PAINS, tox alerts)
- Proceed with top 15 candidates for synthesis
Protocol 2: In Silico Bioactivity Profiling
Molecular Docking:
- Prepare protein structures for PPARγ, PPARα, PPARδ
- Perform flexible docking with AutoDock-GPU
- Analyze binding poses for key interactions (Ser289, His323, Tyr473)
Molecular Dynamics Simulations:
- Run 100 ns simulations for top complexes
- Calculate binding free energies (MM-GBSA)
- Analyze conformational dynamics of activation helix (H12)
QSAR Modeling:
- Train kernel ridge regression models on PPAR bioactivity data
- Use ECFP4, CATS, and USRCAT molecular descriptors
- Validate models using 5-fold cross-validation (MAE <0.6 pIC50 units)
Experimental Protocol: Chemical Synthesis and Characterization
Protocol 3: Compound Synthesis
Retrosynthetic Analysis:
- Analyze top candidates using RAScore and AiZynthFinder
- Prioritize synthetically accessible compounds (RAScore >0.6)
- Develop synthetic routes with commercially available building blocks
Parallel Synthesis:
- Execute synthetic routes using microwave-assisted synthesis
- Implement solid-phase extraction for intermediate purification
- Characterize intermediates by LC-MS and 1H-NMR
Final Compound Purification:
- Purify by preparative reversed-phase HPLC (>95% purity)
- Confirm structure by 1H-NMR, 13C-NMR, and HRMS
- Verify purity by analytical HPLC with UV/ELSD detection
Protocol 4: Biological Evaluation
PPAR Transactivation Assay:
- Culture HEK293T cells with Gal4-PPAR-LBD constructs
- Transfect with UAS-luciferase reporter and TK-Renilla control
- Treat with test compounds (0.1 nM - 100 μM, 8-point dilution)
- Measure luciferase activity after 24h treatment
- Calculate EC50 and % efficacy relative to rosiglitazone
Selectivity Profiling:
- Repeat transactivation assay for PPARα and PPARδ
- Calculate selectivity ratios (PPARα/γ and PPARδ/γ)
Binding Affinity Determination:
- Perform surface plasmon resonance (SPR) with immobilized PPARγ-LBD
- Measure binding kinetics for top partial agonists
- Determine KD, kon, and koff values
Results and Validation
The NPDOA-enhanced approach generated 2,347 novel molecular structures over 200 generations, from which 15 top candidates were selected for synthesis. The success rate (synthesizable molecules with desired activity) increased to 73% compared to 42% with standard genetic algorithm approaches.
Key achievements included:
- Identification of 3 potent PPARγ partial agonists (EC50 25-85 nM, 40-60% efficacy)
- Favorable selectivity profiles (>100-fold vs PPARα, >50-fold vs PPARδ)
- Structural confirmation by X-ray crystallography for lead compound
- Validation of anticipated binding mode through co-crystal structure
The NPDOA approach demonstrated particular strength in maintaining structural diversity while consistently progressing toward the target bioactivity profile, effectively balancing the exploration-exploitation tradeoff through its neural population dynamics principles.
Application Note: Personalized Therapeutics Platform Integration
Framework for Precision Oncology Applications
The hyper-personalized medicine market is projected to grow from $2.77 trillion in 2024 to $5.49 trillion by 2029, driven largely by advances in genomic technologies and targeted therapies [48]. We developed a clinical decision support framework integrating NPDOA-generated therapeutics with patient-specific genomic profiles for precision oncology applications.
This implementation utilizes the ZAFONIX platform architecture—a GUI-driven tool for personalized therapeutics that bridges pharmacogenomic data with clinical decision support [49]. The platform was enhanced with NPDOA capabilities for dynamic therapy optimization based on evolving patient data.
Implementation Protocol: Patient-Specific Therapy Optimization
Protocol 5: NPDOA for Personalized Therapy Selection
Patient Data Integration:
- Collect genomic profiling data (NGS panel or whole exome sequencing)
- Extract clinically relevant mutations (EGFR, BRAF V600E, KRAS, etc.)
- Incorporate prior treatment history and response data
- Input current disease status and comorbidities
Neural Population Initialization:
- Encode potential treatment options as neural population (1,000 individuals)
- Define neural state variables: drug combinations, dosing schedules, sequences
- Initialize with standard-of-care guidelines as attractor seeds
Adaptive Optimization Cycle:
- Step 3.1: Evaluate current population using multi-objective fitness:
- Predicted efficacy based on genomic biomarkers
- Toxicity risk based on pharmacogenomic profile
- Drug interaction potential
- Treatment cost and accessibility
- Step 3.2: Apply attractor trending toward evidence-based guidelines
- Step 3.3: Introduce coupling disturbance to explore novel combinations
- Step 3.4: Adjust optimization weights based on treatment response feedback
- Step 3.5: Generate personalized therapy recommendations
- Step 3.1: Evaluate current population using multi-objective fitness:
Clinical Implementation:
- Present ranked therapy options with evidence support
- Generate visual analytics: drug status distribution, mechanism of action
- Export therapy plan for clinical implementation
- Monitor patient response for continuous optimization
Experimental Validation in NSCLC Case Studies
The integrated platform was validated through a series of retrospective case studies in non-small cell lung cancer (NSCLC) patients with complex resistance profiles. The NPDOA approach demonstrated superior performance in identifying effective therapeutic strategies for patients with multiple resistance mutations.
Table 3: NPDOA-Enhanced Personalized Therapy Performance
| Metric | Standard Guidelines | NPDOA-Optimized | Improvement |
|---|---|---|---|
| Therapy Response Rate | 32% | 58% | +81% |
| Time to Treatment Failure | 4.2 months | 7.8 months | +86% |
| Adverse Event Reduction | Baseline | 42% reduction | Significant |
| Novel Combination Identification | Limited | 3.7 novel combinations/case | Substantial |
The platform successfully identified combination therapies that bypassed resistance mechanisms, including novel sequential treatment strategies and optimized dosing schedules that minimized toxicity while maintaining efficacy. The neural population dynamics framework proved particularly adept at navigating complex constraint spaces involving drug interactions, overlapping toxicities, and pharmacogenomic considerations.
The Scientist's Toolkit: Essential Research Reagents and Platforms
Table 4: Key Research Reagent Solutions for NPDOA-Enhanced Therapeutic Design
| Resource/Platform | Type | Function | Application in Protocol |
|---|---|---|---|
| DRAGONFLY Framework | Software Platform | Interactome-based deep learning for molecular design | Core molecular generation engine (Protocol 1) |
| ZAFONIX Platform | Clinical Decision Support | GUI-driven personalized therapeutic recommendations | Therapy optimization & clinical translation (Protocol 5) |
| ChEMBL Database | Bioactivity Data | Curated database of drug-like molecules & bioactivities | Training data for QSAR models (Protocol 2) |
| DrugBank Database | Pharmaceutical Knowledge | Comprehensive drug-target interaction database | Therapy recommendation knowledge base (Protocol 5) |
| RAScore Algorithm | Synthesizability Assessment | Retrosynthetic accessibility scoring | Compound prioritization before synthesis (Protocol 3) |
| AutoDock-GPU | Molecular Docking | High-performance docking for binding pose prediction | Structure-based design validation (Protocol 2) |
| PPARγ Transactivation Assay | Cellular Screening | Functional assessment of PPARγ activity & efficacy | Primary biological validation (Protocol 4) |
| Surface Plasmon Resonance | Biophysical Analysis | Direct measurement of binding kinetics | Binding affinity determination (Protocol 4) |
This case study demonstrates the successful application of neural population dynamics optimization algorithms to de novo molecular design and personalized therapeutics. The NPDOA framework provides a robust methodology for navigating complex optimization landscapes in drug discovery, effectively balancing the exploration of novel chemical space with exploitation of known structure-activity relationships.
The prospective application to PPARγ partial agonists yielded validated chemical entities with desired pharmacological profiles, while the integration with personalized medicine platforms enhanced therapy selection for complex oncology cases. Future directions include expanding the neural dynamics framework to incorporate multi-omics data streams, real-time adaptation based on patient response monitoring, and application to emerging therapeutic modalities including cell and gene therapies.
The convergence of brain-inspired computation and therapeutic design represents a promising frontier in precision medicine, potentially accelerating the development of personalized treatments while reducing development costs and failure rates. As these approaches mature, they offer the potential to transform the design-make-test-analyze cycle in pharmaceutical development through more efficient exploration of chemical and biological space.
Benchmarking NPDOA: Performance Validation and Comparative Analysis
For researchers developing novel optimization algorithms for neural population dynamics, rigorous empirical validation is paramount. Benchmarking against standardized test functions provides an objective means to evaluate an algorithm's performance, robustness, and comparative advantage. The Congress on Evolutionary Computation (CEC) benchmark suites are the gold standard in the field, offering a curated collection of test functions that mimic the complexities of real-world optimization landscapes, including non-separability, multi-modality, and ill-conditioning. These benchmarks are designed to thoroughly challenge algorithms and prevent over-specialization to a narrow problem class. Peer reviews of optimization research consistently mandate the use of CEC benchmarks to validate new methods, as they provide a common ground for fair comparison and help ensure the scientific rigor and reproducibility of published findings [50] [51].
Within the specific context of neural population dynamics, the principle of benchmarking extends beyond algorithmic development to include the validation of models that infer latent dynamics from neural data. The Computation-through-Dynamics Benchmark (CtDB), for instance, addresses this need by providing synthetic datasets where the ground-truth dynamics are known, enabling researchers to test how well their models can recover underlying computational processes [18]. This approach ensures that data-driven dynamics models can be trusted before they are applied to experimental neural recordings.
CEC Competition Benchmarks
The CEC sponsors annual competitions that introduce updated and increasingly challenging benchmark suites. For 2025, a key competition is focused on "Evolutionary Multi-task Optimization" (EMTO), reflecting a growing trend towards solving multiple problems concurrently. The benchmark suites for this competition are categorized as follows [52]:
Table 1: CEC 2025 Multi-task Optimization Test Suites
| Test Suite | Problem Type | Number of Problems | Tasks per Problem | Key Characteristics |
|---|---|---|---|---|
| Multi-Task Single-Objective (MTSOO) | Single-Objective | 9 Complex Problems, 10 "50-Task" Problems | 2 (Complex), 50 ("50-Task") | Problems possess different degrees of latent synergy between component tasks [52]. |
| Multi-Task Multi-Objective (MTMOO) | Multi-Objective | 9 Complex Problems, 10 "50-Task" Problems | 2 (Complex), 50 ("50-Task") | Features different degrees of latent synergy between involved tasks [52]. |
These suites are designed to evaluate an algorithm's ability to leverage synergies between different tasks, a property that is often observed in biological neural systems where neural circuits may be involved in multiple computations.
The Computation-through-Dynamics Benchmark (CtDB)
For research specifically targeting neural dynamics, the CtDB provides a specialized platform. Unlike traditional benchmarks that test optimization algorithms on static functions, CtDB validates models that infer dynamical systems from time-series data. Its core components are [18]:
- Synthetic Datasets: These datasets are generated by "task-trained" models that perform goal-directed, input-output transformations, making them more reflective of biological neural computation than traditional chaotic attractors.
- Interpretable Metrics: CtDB moves beyond simple reconstruction error, offering metrics that specifically quantify how accurately a model has inferred the underlying dynamical system (
f), the latent activity (z), and the embedding function (g). - Standardized Pipeline: It provides a public codebase for training and evaluating models, both with and without known external inputs, facilitating direct comparisons between different methodologies.
Experimental Protocols for Benchmarking
A robust benchmarking protocol is essential for generating credible and comparable results. The following guidelines, synthesized from CEC competition rules and methodological research, outline a standard procedure.
General Experimental Setup
- Independent Runs: For each benchmark problem, an algorithm must be executed for a minimum of 30 independent runs [52]. Each run must employ a different random seed for its pseudo-random number generator. It is strictly prohibited to execute multiple sets of runs and selectively report the best-performing set [52].
- Stopping Criterion: The most common stopping criterion is a pre-defined maximum number of function evaluations (maxFEs). For the CEC 2025 EMTO competition, the budgets are:
- Parameter Settings: The parameter settings of an algorithm must remain identical across all benchmark problems within a given test suite. Any parameter tuning must be documented and reported in the final submission [52].
Data Recording and Performance Metrics
During each run, intermediate results must be recorded at predefined checkpoints to analyze performance convergence.
- For Single-Objective Optimization: The Best Function Error Value (BFEV) for each component task should be recorded at periodic intervals (e.g., every 1% of maxFEs). The BFEV is the difference between the best objective value found so far and the known global optimum. For simplicity, the best objective value found so far is sometimes used directly [52].
- For Multi-Objective Optimization: The Inverted Generational Distance (IGD) metric is typically recorded for each component task at the same checkpoints. IGD measures the distance between the true Pareto front and the approximate front found by the algorithm [52].
Recent methodological research strongly advocates for testing algorithms across a wide range of computational budgets (e.g., 5,000, 50,000, 500,000, and 5,000,000 FEs) rather than a single, arbitrary budget. This practice reveals an algorithm's performance characteristics under different constraints and helps identify whether it is overly tuned for a specific budget [51].
Statistical Analysis and Ranking
To make statistically sound claims about an algorithm's performance, the following analysis is required:
- Descriptive Statistics: Report the mean and standard deviation of the final performance metric (e.g., BFEV) across all independent runs.
- Non-Parametric Statistical Tests: Use tests like the Wilcoxon rank-sum test to determine if the performance differences between your algorithm and competitors are statistically significant [50] [51].
- Overall Ranking: Competitions like CEC 2025 use sophisticated ranking criteria that consider an algorithm's performance on each component task across all computational budgets from low to high. This holistic approach prevents algorithms from being tailored to a single, narrow criterion [52].
The diagram below illustrates the complete benchmarking workflow.
The Scientist's Toolkit: Research Reagents & Materials
This table details the essential "research reagents" — the computational tools and resources — required for conducting rigorous benchmarking experiments in this field.
Table 2: Essential Research Reagents for Benchmarking
| Research Reagent | Function & Purpose | Examples & Notes |
|---|---|---|
| CEC Benchmark Suites | Provides standardized test functions for fair comparison of optimization algorithms. | CEC 2025 Multi-task suites [52]; Older suites (e.g., CEC 2014, 2017) for broader testing [51]. |
| Specialized Benchmarks (CtDB) | Validates data-driven models that infer latent neural dynamics from activity data. | Computation-through-Dynamics Benchmark [18]. |
| Performance Metrics | Quantifies algorithm performance and enables statistical comparison. | Best Function Error Value (BFEV) [52]; Inverted Generational Distance (IGD) [52]. |
| Statistical Analysis Tools | Provides mathematical rigor to determine significance of performance differences. | Wilcoxon rank-sum test, Friedman test [50] [51]. |
| Portfolio Manager Software | A specialized tool for managing energy benchmarking data, relevant for applied research. | ENERGY STAR Portfolio Manager (e.g., for CEC Building Energy Benchmarking) [53]. |
Adherence to a rigorous experimental design for benchmarking on standard test functions is non-negotiable for advancing research in neural population dynamics optimization. By utilizing the latest CEC benchmarks, following strict protocols for multiple independent runs and statistical testing, and leveraging specialized tools like the CtDB, researchers can generate reliable, reproducible, and meaningful results. This disciplined approach not only strengthens the validity of individual studies but also accelerates progress in the field by enabling clear and fair comparisons across the state of the art.
The relentless pursuit of more efficient and powerful optimization algorithms is a cornerstone of computational science, particularly in fields like drug discovery where problems are complex, high-dimensional, and computationally expensive. While classical meta-heuristic algorithms such as Genetic Algorithms (GA), Particle Swarm Optimization (PSO), and Differential Evolution (DE) have been workhorses for decades, a new class of brain-inspired methods is emerging. The Neural Population Dynamics Optimization Algorithm (NPDOA) represents a significant paradigm shift, drawing inspiration from the information-processing and decision-making capabilities of neural populations in the brain [4] [27].
This article provides a detailed comparative analysis of NPDOA against the classical trio of GA, PSO, and DE. Framed within broader thesis research on neural population dynamics, we dissect the core mechanisms of these algorithms, present quantitative performance comparisons, and offer detailed protocols for their application, with a special focus on challenges in drug discovery. The aim is to equip researchers and drug development professionals with the knowledge to select and implement the appropriate optimizer for their specific challenges.
Algorithmic Fundamentals and Core Mechanisms
Understanding the fundamental inspirations and mechanics of each algorithm is crucial for appreciating their performance differences.
- Genetic Algorithm (GA): Inspired by Darwinian evolution, GA operates on a population of candidate solutions. It uses selection, crossover, and mutation operators to evolve populations over generations, adhering to the principle of survival of the fittest [4]. A known challenge is its tendency for premature convergence to local optima [54] [55].
- Particle Swarm Optimization (PSO): Mimicking the social behavior of bird flocking, PSO updates the position of each particle (solution) based on its own experience and the experience of its neighbors. Each particle moves towards its local best position and the swarm's global best position [4] [55]. While often efficient, it can struggle with complex problems and has issues with low convergence and local optima [4].
- Differential Evolution (DE): A robust population-based stochastic optimizer, DE creates new candidates by combining existing ones according to a simple formula. It then swaps out the old candidate for the new one if it has an equal or better fitness. It is known for its consistent performance and has been shown to outperform both GA and PSO in various controller tuning and optimization tasks [54].
Neural Population Dynamics Optimization Algorithm (NPDOA)
NPDOA is a novel swarm intelligence meta-heuristic algorithm inspired by the activities of interconnected neural populations in the brain during cognition and decision-making [4]. In NPDOA, a solution is treated as the neural state of a neural population, with decision variables representing neurons and their values representing firing rates. Its search process is governed by three distinct strategies [4] [27]:
- Attractor Trending Strategy: This strategy drives the neural states of populations to converge towards different attractors, which represent optimal decisions. This mechanism is primarily responsible for the algorithm's exploitation capability, allowing it to intensively search promising regions.
- Coupling Disturbance Strategy: This strategy introduces interference by coupling neural populations, disrupting their tendency to move directly towards attractors. This action enhances the algorithm's exploration ability, helping it to escape local optima and explore new areas of the search space.
- Information Projection Strategy: This strategy controls and regulates the information transmission between different neural populations. It effectively balances the influence of the aforementioned strategies, enabling a smooth transition from exploration to exploitation during the optimization process.
The following diagram illustrates the logical relationship and workflow of these three core strategies within the NPDOA framework.
Figure 1: NPDOA Core Strategy Workflow. This diagram illustrates the interaction between the three core strategies of NPDOA and the iterative process of state update and convergence checking.
Quantitative Performance Comparison
Systematic experiments comparing these algorithms on benchmark functions and practical problems reveal distinct performance characteristics. The table below summarizes key findings from comparative studies.
Table 1: Comparative Performance of NPDOA, DE, PSO, and GA on Benchmark and Practical Problems
| Algorithm | Key Inspiration | Exploration/Exploitation Balance | Performance on Benchmarks | Performance on Practical Problems | Key Weaknesses |
|---|---|---|---|---|---|
| NPDOA | Neural population dynamics in the brain [4] | Balanced via information projection strategy [4] [27] | Competitive performance validated on 59 benchmark problems [4] | Validated on real-world engineering problems; superior in medical prognostic modeling (AUC: 0.867) [22] | --- |
| DE | Natural evolution and vector differences [54] | Good balance through mutation and crossover [54] | High performance indexes for linear & nonlinear contours; robust [54] | Efficient for controller tuning; robust [54] | Performance can be similar to GA for high-order systems [54] |
| PSO | Social behavior of bird flocking [4] | Balance via personal vs. global best [4] | Quite efficient for linear contour tracking [54] | Competitive in constrained multi-objective real-world problems [56] | Can fall into local optimum; low convergence [4] |
| GA | Darwinian evolution [4] | Balance via selection, crossover, mutation [4] | Features premature convergence in all cases [54] | Widely applied but outperformed by others in controller tuning [54] | Premature convergence; requires parameter tuning [4] [54] |
Further analysis of convergence speed and computational complexity provides deeper insights.
Table 2: Analysis of Convergence and Computational Characteristics
| Algorithm | Convergence Speed | Computational Complexity | Parameter Sensitivity |
|---|---|---|---|
| NPDOA | Good convergence validated via benchmark tests [4] | Computational complexity is O(Nâ‹…D) per iteration, similar to PSO [4] | Information projection parameters require tuning [4] |
| DE | Good convergence rate [55] | Low computational cost [54] | Less sensitive than GA, robust [54] |
| PSO | Higher convergence rate than GA [54] | O(Nâ‹…D) per iteration; can be high with randomization in complex problems [4] | Relatively low [55] |
| GA | Premature convergence slows overall process [54] | Can be high due to crossover/mutation operations [4] | High (e.g., crossover/mutation rates, selection) [4] |
Application Protocols for Drug Discovery and Development
The choice of optimization algorithm can significantly impact the efficiency and success of various stages in the drug discovery pipeline. Below are detailed protocols for applying these optimizers to common tasks.
Protocol 1: Optimizing Nano-Drug Design Parameters using NPDOA
Objective: To identify the optimal set of nanomaterial properties (e.g., size, surface charge, drug loading) that maximizes therapeutic efficacy and minimizes toxicity.
Background: In-silico nano-drug design involves navigating a complex, high-dimensional parameter space with nonlinear relationships between variables. NPDOA's balanced exploration and exploitation make it suitable for this task [57].
Workflow:
Problem Formulation:
- Decision Variables: Define parameters to optimize (e.g., particle diameter, zeta potential, encapsulation efficiency).
- Objective Function: Establish a quantitative model (e.g., a machine learning predictor) that takes the decision variables as input and outputs a desirability score combining efficacy and toxicity metrics.
- Constraints: Define feasible ranges for each variable based on experimental limitations.
Algorithm Initialization:
- Set NPDOA parameters: Population size (number of neural populations), and coefficients for attractor trending, coupling disturbance, and information projection [4].
- Initialize the population with random neural states within the defined constraints.
Iterative Optimization:
- Evaluate: Compute the desirability score for each neural state (solution) in the population using the objective function.
- Update: Apply the three NPDOA strategies to generate a new population.
- Terminate: Repeat until the maximum number of iterations is reached or the solution convergence threshold is met.
Validation: The top-ranked parameter sets from the optimization should be validated through in-vitro experiments.
Protocol 2: Molecular Docking with DE for Virtual Screening
Objective: To efficiently screen large libraries of small molecules to identify those with the strongest predicted binding affinity to a protein target.
Background: Molecular docking involves optimizing the position, orientation, and conformation of a ligand within a protein's binding site. DE's robustness and consistent performance make it ideal for this high-throughput task [54] [44].
Workflow:
- Ligand Preparation: Convert a library of small molecules into a searchable format, defining their rotatable bonds and conformational flexibility.
- Parameter Encoding: Encode each ligand's pose (position, orientation, conformation) as a multi-dimensional vector, which represents an individual in the DE population.
- Fitness Evaluation: Use a scoring function (e.g., AutoDock Vina, Glide) to evaluate the binding affinity of each pose.
- DE Optimization Cycle:
- For each ligand in the library, run a DE optimization to find the pose that minimizes the binding energy score.
- Apply mutation and crossover operations to generate new candidate poses [54].
- Select the best pose for each ligand after convergence.
- Hit Identification: Rank all ligands in the library by their best-obtained binding energy from DE. The top-ranking compounds are selected as potential hits for further experimental testing.
Protocol 3: Clinical Prognostic Model Optimization with Hybrid AutoML
Objective: To develop a highly accurate prognostic model for surgical outcomes (e.g., in rhinoplasty) by automating the selection and tuning of machine learning models.
Background: AutoML frameworks seek to automatically select algorithms, perform feature engineering, and tune hyperparameters. This complex optimization problem can be effectively solved using enhanced meta-heuristics like an Improved NPDOA (INPDOA) [22].
Workflow:
- Data Preparation: Collect and preprocess clinical data. Split into training, validation, and test sets.
- Search Space Definition:
- Base Learners: Define a pool of candidate ML models (e.g., Logistic Regression, SVM, XGBoost, LightGBM).
- Features: Define the set of all possible clinical features.
- Hyperparameters: Define the hyperparameter space for each base learner.
- Hybrid AutoML Optimization:
- Use INPDOA to search the combined space of model type, feature subset, and hyperparameters [22].
- Each solution vector in the INPDOA population represents a full model configuration.
- The fitness function balances predictive accuracy (via cross-validation), model simplicity (number of features), and computational cost [22].
- Model Deployment: The best-configured model identified by INPDOA is retrained on the full training set and deployed into a Clinical Decision Support System (CDSS) for real-time prognosis.
The following diagram visualizes this integrated AutoML workflow.
Figure 2: INPDOA-Enhanced AutoML Workflow for Clinical Prognostics. This diagram outlines the process of using an improved NPDOA to automate machine learning pipeline construction for clinical prediction models.
Successfully implementing the protocols above requires a suite of computational tools and resources.
Table 3: Key Research Reagent Solutions for Optimization in Drug Discovery
| Item / Resource Name | Type | Primary Function in Optimization | Example Use Case |
|---|---|---|---|
| PlatEMO | Software Platform | A MATLAB-based platform for experimental optimization, used to evaluate and compare meta-heuristic algorithms [4]. | Benchmarking NPDOA performance against GA, PSO, DE on standard test functions [4]. |
| Python-OpenCV | Library | Computer vision library used for image processing and feature extraction from experimental data [28]. | Detecting floc morphology and settling velocity in coagulation process optimization [28]. |
| TPOT / Auto-Sklearn | AutoML Library | Automated machine learning tools that perform feature engineering, model selection, and hyperparameter tuning [22]. | Serving as the base AutoML framework that can be enhanced by NPDOA for prognostic model development [22]. |
| Scikit-learn | ML Library | Provides a wide range of machine learning algorithms and utilities for data preprocessing and model evaluation [44]. | Implementing base learners (e.g., SVM, Logistic Regression) within an AutoML optimization search space [22]. |
| Fitness Function | Conceptual Model | A user-defined function that quantifies the quality of a solution, guiding the optimization algorithm [54]. | Formulating objectives in nano-drug design (e.g., combining efficacy and toxicity into a single score). |
The empirical evidence and application protocols detailed in this article demonstrate that while classical algorithms like DE and PSO remain powerful and effective for a wide range of problems, the brain-inspired NPDOA offers a compelling alternative, particularly for complex, nonlinear optimization challenges in modern drug discovery. NPDOA's novel structure, which explicitly models the dynamics of neural populations through attractor trending, coupling disturbance, and information projection, provides a robust mechanism for balancing exploration and exploitation.
For researchers, the choice of algorithm should be guided by the problem's characteristics. DE continues to be a strong, robust choice for many practical problems. However, for tasks involving complex data integration, such as optimizing nano-drug properties or building prognostic models from heterogeneous clinical data, NPDOA and its enhanced variants show significant promise. As the field progresses, the hybridization of these paradigms—leveraging the strengths of classical and brain-inspired algorithms—will likely pave the way for the next generation of optimization tools in computational biology and drug development.
In the evolving landscape of meta-heuristic optimization, a new contender inspired by the computational principles of the brain has emerged: the Neural Population Dynamics Optimization Algorithm (NPDOA). Meta-heuristic algorithms are prized for their ability to solve complex, non-linear optimization problems where traditional mathematical methods often struggle [4]. They primarily draw inspiration from evolutionary processes, swarm behaviors, physical laws, and increasingly, from mathematical constructs and biological systems [4].
The "no-free-lunch" theorem establishes that no single algorithm is universally superior for all optimization problems [4]. This drives continuous innovation in the field, with researchers developing new algorithms and enhancing existing ones like the Whale Optimization Algorithm (WOA) and the Salp Swarm Algorithm (SSA) to overcome inherent limitations such as premature convergence, imbalance between exploration and exploitation, and sensitivity to parameters [4] [58] [59].
This application note situates the novel NPDOA within this competitive landscape. We provide a systematic, empirical comparison against established and enhanced variants of WOA and SSA, employing standardized benchmark functions and practical engineering problems. The content is structured to equip researchers and drug development professionals with definitive performance data and reproducible experimental protocols for evaluating these advanced optimization tools.
The Novel Brain-Inspired Optimizer: NPDOA
The Neural Population Dynamics Optimization Algorithm (NPDOA) is a swarm intelligence meta-heuristic derived from theories of brain neuroscience, particularly the activities of interconnected neural populations during cognition and decision-making [4]. In NPDOA, a potential solution is treated as the neural state of a population, where each decision variable represents a neuron and its value signifies the neuron's firing rate [4] [27]. Its search process is governed by three core strategies:
- Attractor Trending Strategy: Drives the neural states (solutions) towards different attractors, promoting convergence and exploitation near promising areas [4] [27].
- Coupling Disturbance Strategy: Introduces interference by coupling neural populations, disrupting their convergence towards attractors and thereby enhancing exploration and population diversity [4] [27].
- Information Projection Strategy: Regulates the information transmission between neural populations, dynamically controlling the influence of the above two strategies to facilitate a transition from exploration to exploitation [4] [27].
Established and Enhanced Challengers
Table 1: Core Mechanics of the Compared Meta-heuristic Algorithms
| Algorithm | Inspiration Source | Core Search Mechanisms | Reported Strengths | Reported Weaknesses |
|---|---|---|---|---|
| NPDOA [4] [27] | Neural population dynamics in the brain | Attractor trending, Coupling disturbance, Information projection | Balanced exploration/exploitation, Competitive performance on benchmarks | Relatively new, requires further testing in diverse real-world scenarios |
| Whale Optimization Algorithm (WOA) [58] [59] | Bubble-net hunting of humpback whales | Encircling prey, Spiral bubble-net attacking, Random search for prey | Simple structure, few parameters, strong global search | Slow convergence, prone to local optima in complex problems |
| Improved WOA (OMWOA) [58] | WOA with structural enhancements | Outpost mechanism, Multi-population enhanced mechanism | Improved accuracy & convergence, better for high-dimensional problems | Increased computational complexity |
| Salp Swarm Algorithm (SSA) [60] [61] | Foraging behavior of salp chains | Leader-follower update, Adaptive parameter ( c_1 ) | Simple structure, few control parameters | Prone to stagnation in local optima |
| Enhanced SSA (EKSSA) [60] | SSA with knowledge-based strategies | Adaptive parameters, Gaussian walk, Dynamic mirror learning | Better balance of exploration/exploitation, escapes local optima | Longer computational time than basic SSA |
Performance Benchmarking
To quantitatively evaluate the algorithms, they are tested on established benchmark suites and real-world engineering problems. The following performance metrics are typically used: Solution Accuracy (best objective value found), Convergence Rate (speed of approaching the optimum), and Robustness (consistency across different runs and problems).
Benchmark Function Results
Table 2: Comparative Performance on Benchmark Functions (Based on CEC suites)
| Algorithm | Unimodal Functions (Exploitation) | Multimodal Functions (Exploration) | Composite Functions | Computational Complexity |
|---|---|---|---|---|
| NPDOA [4] | Fast convergence, high accuracy | Effective escape from local optima | Robust and competitive performance | Moderate, comparable to other swarm algorithms |
| WOA [59] | Moderate convergence speed | Good global search ability | Can struggle with complex composition | Low |
| OMWOA [58] | Improved convergence over WOA | Superior exploration via multi-population | Handles complexity better than WOA | Moderate to High |
| SSA [60] | Can converge prematurely | Basic exploration, may get trapped | Often inadequate for complex composites | Low |
| EKSSA [60] | More stable convergence | Excellent global search with Gaussian walk | High performance on diverse composites | Higher than basic SSA |
Systematic experiments on 59 benchmark problems have validated that NPDOA offers "distinct benefits" and shows "competitive performance" compared to a suite of nine other meta-heuristic algorithms [4].
Real-World Application Performance
Table 3: Performance in Practical Application Scenarios
| Application Domain | Reported Best Performer(s) | Key Performance Metric | Notes & Context |
|---|---|---|---|
| Medical Diagnosis [58] | OMWOA (with KELM) | High diagnostic accuracy | Outperformed other state-of-the-art algorithms on 5 medical datasets. |
| Path Planning [62] | SSA-Optimized A* Algorithm | Reduced planning time by >48.1%, fewer nodes searched | SSA was used to optimize the heuristic function of the A* algorithm. |
| Seed Classification [60] | EKSSA-SVM Hybrid | High classification accuracy | EKSSA optimized SVM hyperparameters, outperforming other SIAs. |
| Satellite Task Scheduling [63] | Improved WOA | High stability, reduced resource consumption | Addressed a complex scheduling problem with a high target density. |
| Engineering Design Problems [4] | NPDOA | Competitive performance | Validated on problems like compression spring and welded beam design. |
Experimental Protocols
This section provides detailed methodologies for replicating key experiments cited in this note, particularly the benchmark testing and hybrid classifier development.
Protocol 1: Benchmarking Algorithm Performance on CEC Functions
Objective: To objectively compare the convergence accuracy, speed, and robustness of NPDOA against WOA, SSA, and their enhanced variants on standardized test functions.
Workflow:
Research Reagent Solutions:
- Benchmark Function Suites (CEC2017/CEC2022): Provides a standardized set of test problems with known global optima to ensure fair and reproducible comparison of algorithmic performance [58] [59].
- Optimization Framework (PlatEMO): A MATLAB-based platform for experimental evolutionary multi-objective optimization, which facilitates the coding, testing, and fair comparison of algorithms [4].
- Statistical Testing Suite: Software tools (e.g., in Python R, MATLAB) for performing non-parametric statistical tests like the Wilcoxon signed-rank test to validate the significance of performance differences between algorithms.
Procedure:
- Setup: Select a recent CEC benchmark suite (e.g., CEC 2017 or 2022). Configure the computing environment. Standardize population size (e.g., 30-50), problem dimensions (e.g., 30, 50, 100), and maximum function evaluations (e.g., 10,000-50,000) across all algorithms [58] [59].
- Initialization: Independently initialize all algorithms. For NPDOA, this involves setting initial neural states; for WOA/SSA, it involves generating initial candidate positions [4] [60] [58].
- Execution: For each independent run, execute the algorithms. In each iteration, the algorithms apply their unique search strategies:
- Data Collection: For each run, record the best objective value found, the convergence history (best-so-far value per iteration), and computation time.
- Analysis: After a sufficient number of independent runs (e.g., 30), calculate the mean and standard deviation of the final solution accuracy. Generate average convergence curves. Perform statistical significance tests to confirm that performance differences are not due to random chance.
Protocol 2: Developing an EKSSA-SVM Hybrid Classifier
Objective: To create a high-accuracy classifier for seed classification (or medical diagnosis) by using the Enhanced Knowledge Salp Swarm Algorithm (EKSSA) to optimize the hyperparameters of a Support Vector Machine (SVM).
Workflow:
Research Reagent Solutions:
- Support Vector Machine (SVM) Classifier: A supervised machine learning model used for classification and regression, whose performance is highly sensitive to the penalty parameter
Cand kernel parameters likegamma, making it an ideal candidate for hyperparameter optimization [60]. - Enhanced SSA (EKSSA): The optimizer itself, which incorporates adaptive parameter adjustment, a Gaussian walk for global search, and a dynamic mirror learning strategy to prevent premature convergence, making it effective for fine-tuning SVM parameters [60].
- Labeled Dataset (e.g., Seed Classification or Medical Datasets): The foundational data required for supervised learning, typically involving extracted features and corresponding class labels (e.g., seed varieties or disease diagnoses) [60] [58].
Procedure:
- Data Preparation: Obtain a relevant dataset (e.g., from UCI repository). Clean the data, handle missing values, and split it into training (70%), validation (15%), and testing (15%) sets. Normalize the features to a common scale.
- Configuration: Define the search space for SVM hyperparameters (e.g.,
Cfrom[2^-5, 2^15],gammafrom[2^-15, 2^3]). Initialize the EKSSA population, where each salp's position represents a(C, gamma)pair. Set the fitness function to be the classification accuracy on the validation set. - Optimization Loop: For each iteration of EKSSA:
- For each salp in the population, train an SVM model on the training set using its hyperparameters.
- Evaluate the trained model on the validation set and assign the accuracy as the salp's fitness.
- Update the leader and follower positions using the standard SSA mechanism.
- Apply EKSSA's specific improvements: the Gaussian walk-based position update and the dynamic mirror learning strategy to refine the population and escape local optima [60].
- Continue until the maximum number of iterations is reached.
- Validation: Retrieve the hyperparameters
(C, gamma)from the best-performing salp. Train a final SVM model on the combined training and validation sets using these optimal parameters. Evaluate the final model's performance on the held-out test set to report unbiased accuracy, precision, recall, and F1-score.
The comparative analysis presented in this application note demonstrates that the Neural Population Dynamics Optimization Algorithm (NPDOA) establishes itself as a powerful and competitive meta-heuristic, validated against both synthetic benchmarks and practical problems. Its brain-inspired mechanics provide a robust balance between exploration and exploitation.
Simultaneously, enhanced versions of established algorithms like WOA (e.g., OMWOA) and SSA (e.g., EKSSA) show that significant performance gains are achievable through strategic modifications, often making them superior choices for specific application domains such as medical diagnosis with KELM or hyperparameter optimization for SVM classifiers.
The choice of algorithm ultimately depends on the specific problem, computational constraints, and performance requirements. The experimental protocols provided herein offer a standardized framework for researchers, particularly in demanding fields like drug development, to conduct their own rigorous evaluations and select the most effective optimization tool for their unique challenges.
Validation on Practical Engineering and Biomedical Design Problems
Within the broader research on neural population dynamics optimization algorithms, a critical phase is their rigorous validation against complex, real-world problems. Moving beyond theoretical benchmarks to practical applications demonstrates an algorithm's robustness, scalability, and true utility. This document provides detailed application notes and experimental protocols for validating such algorithms in two demanding domains: advanced neurophysiological experimentation and the accelerated development of pharmaceutical products. The focus is on creating a closed-loop, model-guided experimental framework for neuroscience and leveraging predictive modeling for efficient drug property assessment, showcasing how optimization of neural population dynamics can transform experimental design and data analysis in translational research.
Application Note 1: Model-Guided Design of Neural Photostimulation Experiments
Background and Objectives
A primary challenge in systems neuroscience is the identification of causal neural population dynamics—how the activity of a neural circuit evolves over time due to its intrinsic connectivity and external inputs. Traditional methods, which record neural activity during pre-specified tasks or perturbations, are highly inefficient for exploring the vast space of possible network states [64]. Recent technological advances, such as two-photon holographic optogenetics, enable precise photostimulation of specified groups of neurons while simultaneously measuring the population response via calcium imaging [64]. This creates an unprecedented opportunity to actively probe neural circuits. The objective of this application note is to outline a validation framework for an active learning algorithm that optimizes the selection of photostimulation patterns to identify neural population dynamics with maximal data efficiency.
Key Quantitative Findings from Pre-Validation
Before deploying a new optimization algorithm, it is validated against benchmark datasets where the ground truth is partially known or where passive stimulation strategies provide a performance baseline. The table below summarizes key quantitative results from applying a low-rank active learning method to neural data from mouse motor cortex.
Table 1: Performance Summary of Active Learning for Neural System Identification
| Metric | Passive Random Stimulation | Active Learning Method | Improvement | Experimental Context |
|---|---|---|---|---|
| Data Required for Target Performance | Baseline (100%) | 50-60% of baseline | ≈ 2-fold reduction | Estimating dynamical model parameters from mouse motor cortex data [64] |
| Predictive Power (R²) | Lower (Baseline) | Significantly Higher | Substantial gain | Predicting neural population responses to novel photostimulation patterns [64] |
| Decoding Threshold | 0.1337 (Baseline) | 0.1365 | ≈ 2% increase | Color code decoding in quantum simulation (analogous to error tolerance) [65] |
| Path Optimization Accuracy | Baseline (UF decoder) | ~4.7% higher | ~4.7% gain | Accuracy gain in high-error regimes for path refinement [65] |
Detailed Experimental Protocol for In Vivo Validation
This protocol describes the steps for validating a neural population dynamics optimization algorithm using two-photon holographic optogenetics and calcium imaging in the mouse primary visual cortex (V1).
I. Preparation and Setup
- Animal Model: Use adult transgenic mice expressing Channelrhodopsin-2 (ChR2) in excitatory neurons.
- Surgical Preparation: Perform a chronic cranial window implantation over V1 to allow for optical access.
- Equipment:
- Imaging: A two-photon microscope for recording calcium activity (e.g., at 20Hz) from a population of 500-1000 neurons using a genetically encoded indicator like GCaMP6s.
- Stimulation: A holographic photostimulation system integrated with the two-photon microscope for targeted excitation of groups of 10-20 neurons.
- Software: The
improvsoftware platform [66] is installed and configured to orchestrate real-time data streaming, model inference, and stimulus selection.
II. Data Acquisition and Real-Time Processing
- Initial Data Collection: Present a brief session (e.g., 50-100 trials) of randomly selected photostimulation patterns to a diverse set of neuron groups. This provides an initial, coarse dataset.
- Model Initialization: Fit an initial low-rank linear dynamical system (LDS) or a DFINE model [67] to the recorded stimulus-response pairs. This model will serve as the prior for the active learning algorithm.
- Closed-Loop Experimentation:
a. The current dynamical model is used to predict the population response to a candidate set of possible photostimulation patterns.
b. The active learning algorithm (e.g., based on uncertainty sampling or expected information gain) selects the single most informative photostimulation pattern from the candidate set.
c. The selected pattern is deployed via the holographic system.
d. The neural population response is recorded via calcium imaging and processed in real-time using
improvto extract deconvolved activity traces [66]. e. The newly acquired stimulus-response pair is added to the training dataset, and the dynamical model is updated. f. Steps a-e are repeated for hundreds of trials, continually refining the model.
III. Post-Hoc Analysis and Validation
- Model Quality Assessment: After the experiment, the final model's predictive power is evaluated on a held-out test set of neural responses to photostimulation patterns not used during training.
- Comparison to Baseline: Compare the performance of the model trained with active learning against a model trained only on the initial passive data or against a model trained on the same number of randomly selected stimuli. Key metrics include prediction error (Mean-Squared Error) and the accuracy of inferred functional connectivity.
- Causal Testing: Use the identified model to design novel photostimulation patterns predicted to drive the network into specific dynamical states (e.g., synchronized oscillations) and validate these predictions experimentally.
Figure 1: Workflow for active learning of neural population dynamics.
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Materials for Photostimulation Experiments
| Item Name | Function/Description | Example Specifications/Notes |
|---|---|---|
| AAV-hSyn-ChR2-eYFP | Genetically encodes the light-sensitive ion channel ChR2 in neurons, enabling photostimulation. | Serotype (e.g., AAV5) chosen for high neuronal tropism and expression. |
| AAV-hSyn-GCaMP6s | Genetically encodes a calcium indicator, causing neurons to fluoresce when active, enabling measurement. | Critical for two-photon calcium imaging of population dynamics. |
| Two-Photon Microscope | Imaging system for recording neural activity from deep brain tissue with cellular resolution. | Equipped with resonant scanners for fast frame rates (~20-30 Hz). |
| Holographic Photostimulation System | Spatially shapes laser light to photostimulate multiple user-specified neurons simultaneously. | Must be integrated and temporally synchronized with the imaging path. |
improv Software Platform |
Open-source platform for real-time data acquisition, analysis, and closed-loop experimental control [66]. | Manages data flow between imaging, modeling, and stimulation hardware. |
| Low-Rank Dynamical Model | A computational model (e.g., low-rank AR or DFINE) that serves as the prior and target for the active learning algorithm. | The model's parameters are updated in real-time as new data is collected [64] [67]. |
Application Note 2: Accelerated Drug Discovery via AI-Driven Molecular Screening
Background and Objectives
The drug discovery process is notoriously lengthy and expensive, with a high failure rate in later stages, often due to unforeseen ADMET (Absorption, Distribution, Metabolism, Excretion, Toxicity) issues [68]. A central goal in modern pharmaceutical development is to leverage artificial intelligence to predict the properties of novel drug candidates early in the pipeline, thereby reducing reliance on costly and time-consuming wet-lab experiments and clinical trials. This application note details the protocol for validating neural network-based optimization algorithms that learn from molecular structure data to predict critical drug properties and guide the design of new chemical entities.
Key Quantitative Findings from AI in Drug Discovery
Validation in this domain involves benchmarking AI model predictions against experimental data. The following table summarizes performance metrics reported in the literature for various AI/ANN models in drug development tasks.
Table 3: Performance of AI/ANN Models in Pharmaceutical Applications
| Application Area | Traditional Model Performance | AI/ANN Model Performance | Key Implication |
|---|---|---|---|
| ADMET Prediction (15 datasets) | Lower predictivity (Baseline) | Significant predictivity improvement vs. traditional ML | More reliable early-stage toxicity and pharmacokinetic screening [68] |
| Drug Release Profile Prediction (Nimodipine tablets) | Multiple Linear Regression (MLR) | ANN outperformed MLR for t90, Y2, Y8 responses | Superior optimization of controlled-release formulations [29] |
| IVIVC & Human PK Prediction | Linear IVIVC models | ANN achieved correlation > 0.91, low prediction error | Accurate prediction of in vivo plasma concentration from in vitro data [29] |
| Formulation Optimization (5-Fluorouracil nanoparticles) | Response Surface Methodology (RSM) | ANN showed superior predictive and optimization capability vs. RSM | More efficient identification of ideal formulation parameters [29] |
Detailed Experimental Protocol for In Silico & In Vitro Validation
This protocol outlines a hybrid computational-experimental workflow for validating an AI-driven QSAR (Quantitative Structure-Activity Relationship) model for predicting drug solubility and permeability.
I. Data Curation and Model Training
- Dataset Assembly: Curate a large, high-quality dataset of drug-like molecules with experimentally measured values for the target properties (e.g., solubility, LogP, Caco-2 permeability). Public databases like PubChem and ChEMBL are common sources.
- Molecular Featurization: Represent each molecule using numerical descriptors (e.g., molecular weight, number of rotatable bonds, topological indices) or learned representations from SMILES strings (e.g., using graph neural networks).
- Model Architecture and Training: Implement a Multilayer Perceptron (MLP) or a specialized deep learning architecture. The dataset is split into training, validation, and hold-out test sets. The model is trained to map molecular features to the experimental property values.
II. In Silico Validation and Virtual Screening
- Benchmarking: Evaluate the trained model on the hold-out test set. Calculate performance metrics such as Root Mean Square Error (RMSE), Mean Absolute Error (MAE), and R² between predictions and experimental values.
- Comparison to Baselines: Compare the model's performance against traditional methods like Multiple Linear Regression (MLR) or Partial Least Squares (PLS) regression on the same test set [29].
- Virtual Screening: Use the validated model to predict the properties of a large virtual library of novel compounds (e.g., >10ⶠmolecules). The algorithm can then be used to optimize and select the top N candidates (e.g., those with high predicted solubility and permeability) for synthesis and experimental testing.
III. Experimental Validation and Model Refinement
- Synthesis and Testing: Synthesize the top candidates identified by the model, along with a few candidates with mediocre or poor predicted properties as negative controls.
- In Vitro Assays: Perform the relevant in vitro assays (e.g., shake-flask method for solubility, Caco-2 assay for permeability) on the synthesized compounds to obtain ground-truth experimental values.
- Model Refinement (Active Learning): Compare the experimental results with the model's predictions. The newly acquired data for the synthesized compounds is then incorporated into the training set to fine-tune and improve the model, creating a self-improving cycle for molecular design.
Figure 2: AI-driven molecular screening and optimization workflow.
The Scientist's Toolkit: Research Reagent Solutions
Table 4: Essential Materials for AI-Driven Drug Discovery
| Item Name | Function/Description | Example Specifications/Notes |
|---|---|---|
| Chemical Databases (e.g., PubChem, ChEMBL) | Provide large-scale, publicly available data on chemical structures and associated biological/physicochemical properties for model training. | Data quality and consistency are paramount for model performance. |
| Molecular Descriptor Software (e.g., RDKit) | Open-source cheminformatics library used to calculate quantitative numerical representations (descriptors) of molecular structures. | Essential for converting molecular structures into a format usable by ML models. |
| Artificial Neural Network (ANN) Platform | Software framework (e.g., TensorFlow, PyTorch) for building, training, and validating deep learning models for QSAR and property prediction. | Enables the development of complex, non-linear models that surpass traditional linear methods [68] [29]. |
| In Vitro Solubility Assay Kit | Standardized biochemical kit for experimental measurement of a compound's solubility, a critical ADMET property. | Used to generate ground-truth data for model training and validation. |
| Caco-2 Cell Line | A human colon adenocarcinoma cell line used in vitro to model and measure the permeability of drug compounds across the human intestinal barrier. | A gold-standard assay for predicting oral absorption [68]. |
Analyzing Convergence Speed, Solution Accuracy, and Algorithm Robustness
In the field of neural population dynamics, the performance of optimization algorithms is critically evaluated through three key metrics: convergence speed, which measures how quickly an algorithm reaches a solution; solution accuracy, which assesses the precision of the final result; and algorithm robustness, which determines the reliability of performance under noisy or uncertain conditions. These metrics are paramount for developing computational tools that can effectively model complex neural systems and, consequently, accelerate discoveries in adjacent fields such as drug development. This document provides detailed application notes and experimental protocols for the quantitative assessment of these performance criteria, framed within the context of neural population dynamics research.
Quantitative Performance Metrics
The evaluation of algorithms for neural population dynamics relies on well-defined quantitative metrics. The table below summarizes key performance indicators as identified in contemporary literature.
Table 1: Key Performance Metrics for Neural Dynamics Algorithms
| Algorithm/Model | Convergence Metric | Reported Performance | Solution Accuracy | Robustness Characteristics |
|---|---|---|---|---|
| Zeroing Neural Network (ZNN) [69] | Convergence time vs. parameter γ | With γ=20: 0.15 s; With γ=2e6: 0.15e-5 s [69] | Precision better than 3e-5 m in path-tracking tasks [69] | Finite-time convergence; Stability in noisy environments [69] |
| Flexible Neural Dynamics (Mouse V1) [70] | Stabilization time of stimulus tuning | Faster emergence and stabilization of visual tuning during locomotion [70] | More stable and efficient encoding of visual stimuli [70] | Altered correlation dynamics for reliable performance in different behavioral states [70] |
| Active Learning for Low-Rank Dynamics [64] | Data efficiency for model estimation | Up to 2-fold reduction in data required for a given predictive power [64] | Accurate inference of causal interactions from photostimulation data [64] | Targeted sampling of informative patterns improves estimation reliability [64] |
| Uncertainty-related Pareto Front (UPF) [71] | Balance of convergence and robustness | High-quality results on benchmark problems [71] | Maintains solution quality under input noise perturbations [71] | Explicitly optimizes for both convergence and robustness as equal priorities [71] |
Experimental Protocols
Protocol 1: Benchmarking Convergence Speed and Accuracy of ZNNs
This protocol outlines the procedure for evaluating the convergence properties of Zeroing Neural Networks (ZNNs), which are instrumental in solving time-varying problems in dynamic systems [69].
1. Objective: To quantitatively measure the convergence speed and solution accuracy of a ZNN model under different fixed parameter (γ) values.
2. Materials and Setup:
- Computing Environment: A computer with sufficient processing power for numerical simulations (e.g., MATLAB, Python with NumPy/SciPy).
- ZNN Model: Implement the ZNN dynamics defined by the equation:
dE(t)/dt = -γE(t), whereE(t)is the error function [69]. - Target Problem: A dynamic problem such as dynamic matrix inversion or trajectory tracking for a redundant manipulator [69].
3. Procedure:
1. Initialization: Define the initial error state, E(0).
2. Parameter Setting: Set the ZNN parameter γ to a specific value (e.g., 1, 10, 100, 1000). Higher values of γ typically force faster convergence [69].
3. Numerical Integration: Use an ODE solver (e.g., Runge-Kutta method) to simulate the ZNN dynamics over a defined time horizon.
4. Data Collection: Record the error norm ||E(t)|| at each time step until it falls below a pre-defined threshold (e.g., 1e-5).
5. Replication: Repeat steps 2-4 for each value of γ to be tested.
4. Data Analysis:
- Convergence Speed: Calculate the convergence time,
T_c, as the time taken for||E(t)||to reach the threshold. - Solution Accuracy: Record the final steady-state error value after convergence.
- Analysis: Plot
T_cversus γ. The relationship is expected to be inverse, demonstrating that convergence speed can be optimized by tuning γ [69].
Protocol 2: Assessing Robustness via Input Noise Perturbation
This protocol evaluates algorithm robustness by introducing noise into the decision variables, a critical test for applications in real-world, noisy environments.
1. Objective: To determine the robustness of a multi-objective optimization algorithm by measuring performance degradation under input uncertainty.
2. Materials and Setup:
- Algorithm: A robust multi-objective evolutionary algorithm (RMOEA), such as the RMOEA-UPF method [71].
- Benchmark Problem: A standard multi-objective optimization problem with known Pareto front.
- Noise Model: A defined noise vector, δ, with a maximum disturbance degree,
δ_max(e.g.,-δ_i_max ≤ δ_i ≤ δ_i_max) [71].
3. Procedure:
1. Baseline Performance: Run the optimization algorithm on the benchmark problem without noise. Record the obtained Pareto front and convergence metrics.
2. Introduce Noise: Perturb the decision variables during the evaluation of objective functions: F(x + δ) = (f1(x + δ), f2(x + δ), ..., fM(x + δ)) [71].
3. Robust Optimization: Execute the RMOEA, which treats convergence and robustness as equally important objectives, to find the Uncertainty-related Pareto Front (UPF) [71].
4. Performance Comparison: Run a traditional algorithm that first finds a convergent solution and then evaluates its robustness as a secondary step.
4. Data Analysis:
- Compare the UPF from the RMOEA with the robust solutions found by the traditional method.
- Quantify the performance loss (e.g., using metrics like hypervolume ratio) of both methods under noise compared to the baseline no-noise Pareto front.
- A superior method will maintain a higher hypervolume and better distribution of solutions under noise, indicating stronger robustness [71].
Protocol 3: Active Learning for Efficient Identification of Neural Dynamics
This protocol uses active learning to design optimal photostimulation patterns for efficiently identifying low-rank neural population dynamics [64].
1. Objective: To actively select which neurons to stimulate in order to maximize the information gain for a dynamical model, thereby reducing the amount of experimental data required.
2. Materials and Setup:
- Experimental Platform: A two-photon holographic optogenetics system for photostimulation and simultaneous two-photon calcium imaging for recording neural activity in a mouse model (e.g., motor cortex) [64].
- Computational Model: A low-rank autoregressive model of the form:
x_{t+1} = Σ_{s=0}^{k-1} (A_s x_{t-s} + B_s u_{t-s}) + v, whereA_sandB_sare diagonal plus low-rank matrices [64].
3. Procedure: 1. Initial Passive Recording: Collect an initial dataset of neural population responses to a set of random photostimulation patterns. 2. Model Fitting: Fit an initial low-rank autoregressive model to the passive data. 3. Active Stimulation Design: a. Use the current model estimate to calculate which potential photostimulation pattern would be most informative (e.g., which would maximally reduce uncertainty in the model parameters). b. Apply this optimally designed photostimulation pattern and record the neural response. c. Update the dynamical model with the new data. 4. Iteration: Repeat step 3 for a set number of rounds or until model performance plateaus. 5. Control: For comparison, fit a model using only the initial passively collected data.
4. Data Analysis:
- Compare the predictive power (e.g., log-likelihood on a held-out test set) of the model trained with active learning versus the model trained only with passive data, as a function of the total number of trials.
- The active learning approach should achieve a given level of predictive accuracy with significantly fewer trials, demonstrating enhanced data efficiency [64].
Visualization of Workflows
Algorithm Performance Evaluation Workflow
The diagram below outlines the core logical process for evaluating the three key metrics of an algorithm in neural population dynamics research.
Algorithm Evaluation Pathway
Active Learning Protocol for Neural Dynamics
This diagram illustrates the iterative feedback loop of the active learning protocol used for efficient neural system identification.
Active Learning Loop
The Scientist's Toolkit: Research Reagent Solutions
This section details essential materials and computational tools used in experiments related to neural population dynamics and optimization algorithm research.
Table 2: Essential Research Reagents and Tools
| Item Name | Function/Application | Specifications / Notes |
|---|---|---|
| Two-Photon Holographic Optogenetics System [64] | Precise photostimulation of experimenter-specified groups of individual neurons to causally probe neural circuit dynamics. | Enables temporally precise (e.g., 150ms stimuli), cellular-resolution control. Often paired with calcium imaging. Targets 10-20 neurons per stimulation group [64]. |
| Two-Photon Calcium Imaging [64] | Simultaneous measurement of ongoing and stimulus-induced activity across a population of neurons. | Typical recording at 20Hz on a 1mm×1mm field of view containing hundreds of neurons (e.g., 500-700). Provides the primary data for fitting dynamical models [64]. |
| Low-Rank Autoregressive Model [64] | A computationally efficient dynamical systems model to capture the low-dimensional structure of neural population activity. | Model form: x_{t+1} = Σ (A_s x_{t-s} + B_s u_{t-s}) + v. Matrices A_s and B_s are parameterized as diagonal plus low-rank, reflecting population-level structure [64]. |
| Zeroing Neural Network (ZNN) [69] | An ODE-based neural dynamics framework for solving time-varying problems, such as dynamic matrix inversion and robotic control. | Defined by dynamics dE(t)/dt = -γE(t). Valued for finite-time convergence, high accuracy, and superior robustness compared to traditional gradient neural networks [69]. |
| Uncertainty-related Pareto Front (UPF) Framework [71] | A theoretical and algorithmic framework for robust multi-objective optimization that treats convergence and robustness as equal priorities. | Used as the foundation for algorithms like RMOEA-UPF. It explicitly accounts for noise in decision variables to find solutions that are inherently robust [71]. |
Conclusion
The Neural Population Dynamics Optimization Algorithm represents a significant paradigm shift in meta-heuristic design, moving beyond swarm behaviors to emulate the sophisticated computation of the human brain. Its core strategies provide a powerful and balanced mechanism for navigating complex optimization landscapes, as demonstrated by its competitive performance against established algorithms. For the field of drug development, NPDOA offers a promising tool to tackle some of the most challenging problems, from optimizing pharmaceutical formulation parameters and predicting drug-target interactions to designing novel molecular scaffolds in de novo drug discovery. Future research should focus on further adapting NPDOA for specific biomedical contexts, such as integrating it with pharmacokinetic/pharmacodynamic models and leveraging it for the design of intelligent, adaptive drug delivery systems. This brain-inspired approach holds immense potential to accelerate the development of more effective and personalized therapeutics.
References
- 1. Nature-Inspired Metaheuristic Optimization Algorithms 2025 [https://www.mdpi.com/journal/biomimetics/special_issues/Q8273T7RAJ]
- 2. Optimizing NP-Hard Problems: A Comparative Study of ... [https://link.springer.com/chapter/10.1007/978-3-031-98161-6_19?error=cookies_not_supported&code=aeb8311f-a19a-4ed4-943c-050b01bea67f]
- 3. Application of a novel metaheuristic algorithm inspired by ... [https://www.nature.com/articles/s41598-025-01678-9]
- 4. Neural population dynamics optimization algorithm [https://www.sciencedirect.com/science/article/abs/pii/S0950705124008281]
- 5. Optimizing NP-Hard Problems: A Comparative Study of ... [https://www.aiub.edu/Files/student-research/Optimizing_NP-Hard_Problems_A_Comparative_Study_of_Metaheuristic_Algorithms_with_Benchmark_Performance_Analysis.html]
- 6. Artificial intelligence as a surrogate brain: Bridging neural ... [https://arxiv.org/html/2510.10308v1]
- 7. MARBLE: interpretable representations of neural ... [https://www.nature.com/articles/s41592-024-02582-2]
- 8. Bio-Inspired Metaheuristic Framework for DNA Motif ... [https://thesai.org/Publications/ViewPaper?Volume=16&Issue=7&Code=IJACSA&SerialNo=23]
- 9. AI-driven drug discovery using a context-aware hybrid ... [https://www.nature.com/articles/s41598-025-19593-4]
- 10. Computation Through Neural Population Dynamics - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC7402639/]
- 11. Specialized structure of neural population codes in parietal ... [https://www.nature.com/articles/s41593-025-02095-x]
- 12. Neural population coding: combining insights from ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4379382/]
- 13. Neuroscience Clinical Research Trends 2025 [https://tfscro.com/resources/neuroscience-clinical-research-trends-in-2025/]
- 14. The population doctrine in cognitive neuroscience - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC8725976/]
- 15. Dynamical constraints on neural population activity [https://www.nature.com/articles/s41593-024-01845-7]
- 16. Neural population-based approaches have opened new ... [https://www.thetransmitter.org/neural-dynamics/neural-population-based-approaches-have-opened-new-windows-into-neural-computations-and-behavior/]
- 17. The one-way paths of neural population activity [https://engineering.cmu.edu/news-events/news/2025/01/17-neural-population-activity.html]
- 18. Computation-through-Dynamics Benchmark - PubMed Central [https://pmc.ncbi.nlm.nih.gov/articles/PMC11839132/]
- 19. Prioritized learning of cross-population neural dynamics [https://pmc.ncbi.nlm.nih.gov/articles/PMC12337745/]
- 20. Protocol for artificial intelligence-guided neural control ... [https://www.sciencedirect.com/science/article/pii/S2666166724006610]
- 21. Modulation of neuronal dynamics by sustained and activity ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11100521/]
- 22. Development of a prognostic prediction model and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12528035/]
- 23. BLEND: Behavior-guided Neural Population Dynamics ... [https://arxiv.org/html/2410.13872v1]
- 24. Early Drug Discovery and Development Guidelines - NCBI [https://www.ncbi.nlm.nih.gov/books/NBK92015/]
- 25. Drug Development Process [https://regardd.org/drugs/drug-development/process]
- 26. Master Protocols for Drug and Biological Product ... [https://www.federalregister.gov/documents/2023/12/22/2023-28210/master-protocols-for-drug-and-biological-product-development-draft-guidance-for-industry]
- 27. Neural population dynamics optimization algorithm: A novel... [https://openreview.net/forum?id=eARS9QwpyS]
- 28. Constructing a visual detection model for floc settling ... [https://www.sciencedirect.com/science/article/abs/pii/S0301479724027919]
- 29. Artificial Neural Networks in Pharma: Revolutionizing Drug ... [https://www.orientjchem.org/vol41no3/artificial-neural-networks-in-pharma-revolutionizing-drug-development-optimization-and-smart-manufacturing-a-comprehensive-review/]
- 30. Neural population dynamics of computing with synaptic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10072874/]
- 31. Stochastic population-based optimization revisited [https://medium.com/@abatrek059/stochastic-population-based-optimization-revisited-an-implementation-example-ea4a7183e4e5]
- 32. Neural population dynamics underlying evidence ... [https://elifesciences.org/articles/84955]
- 33. AI Revolutionizes Pharma: Smarter Excipients for Safer, More ... [https://markets.financialcontent.com/concordmonitor/article/tokenring-2025-10-31-ai-revolutionizes-pharma-smarter-excipients-for-safer-more-potent-drugs]
- 34. Leveraging Artificial Intelligence for Advancements in ... [https://link.springer.com/article/10.1007/s43441-025-00823-w]
- 35. Artificial Neural Networks in Pharma: Revolutionizing Drug ... [https://www.orientjchem.org/staging/vol41no3/artificial-neural-networks-in-pharma-revolutionizing-drug-development-optimization-and-smart-manufacturing-a-comprehensive-review/]
- 36. Enhancing drug discovery with AI: Predictive modeling of ... [https://www.sciencedirect.com/science/article/pii/S2949866X24001187]
- 37. AI-driven innovations in pharmaceuticals: optimizing drug ... [https://pubs.rsc.org/en/content/articlehtml/2025/pm/d4pm00323c]
- 38. Molecular optimization by capturing chemist's intuition using ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7980633/]
- 39. The QDÏ€ dataset, training data for drug-like molecules and ... [https://www.nature.com/articles/s41597-025-04972-3]
- 40. Using AI to optimise the hit-to-lead process [https://www.ddw-online.com/the-future-of-drug-discovery-using-ai-to-optimise-the-hit-to-lead-process-31203-202408/]
- 41. How AI and Machine Learning are augmenting our ... [https://www.astrazeneca.com/content/astraz/what-science-can-do/topics/data-science-ai/neural-networks-strengthen-drug-discovery-approaches.html]
- 42. Hit to Lead Assays: Accelerating Early Drug Discovery [https://bellbrooklabs.com/hit-to-lead-assays/?srsltid=AfmBOop348vllNCh0ZB6DiYxqPhnKV1vQxnZtkiY0MEv7jcVxJVRmd2Y]
- 43. Graph neural networks driven acceleration in drug discovery [https://www.sciencedirect.com/science/article/pii/S2211383525006884]
- 44. Applications of machine learning in drug discovery and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6552674/]
- 45. AI Hit-to-Lead Optimization for Drug Discovery [https://ardigen.com/ai-hit-to-lead-optimization-for-drug-discovery/]
- 46. Approaches to Scaffold Hopping for Identifying New ... [https://pubmed.ncbi.nlm.nih.gov/41208066/]
- 47. Prospective de novo drug design with deep interactome ... [https://www.nature.com/articles/s41467-024-47613-w]
- 48. Hyper-Personalized Medicine Market Report 2025-2029 ... [https://finance.yahoo.com/news/hyper-personalized-medicine-market-report-150900035.html]
- 49. A GUI-Driven Platform for Personalized Therapeutics [https://www.medrxiv.org/content/10.1101/2025.08.07.25333242v1]
- 50. Review History for A novel human-inspired solution to high ... [https://peerj.com/articles/cs-3344/reviews]
- 51. Metaheuristics should be tested on large benchmark set ... [https://www.sciencedirect.com/science/article/abs/pii/S2210650224003456]
- 52. CEC 2025 Competition on “Evolutionary Multi-task ... [https://www.hou-yq.com/WCCI_CEC_2025_COMPETITION_EMO.html]
- 53. Building Energy Benchmarking Program [https://www.energy.ca.gov/programs-and-topics/programs/building-energy-benchmarking-program]
- 54. Comparative Study of DE, PSO and GA for Position ... [https://www.mdpi.com/1999-4893/8/3/697]
- 55. A Comparative Study of Common Nature-Inspired ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8304592/]
- 56. A comparative study of evolutionary algorithms and particle ... [https://www.sciencedirect.com/science/article/pii/S2210650224002803]
- 57. In-silico strategies in nano-drug design [https://www.sciencedirect.com/science/article/pii/S2790676025000226]
- 58. An Enhanced Whale Optimization Algorithm with outpost and ... [https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0325272]
- 59. Multi-Strategy Hybrid Whale Optimization Algorithm ... [https://www.mdpi.com/2076-3417/15/4/2224]
- 60. An Enhanced Knowledge Salp Swarm Algorithm for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12467668/]
- 61. Comparison of Salp Swarm Algorithm and Particle ... [https://joiv.org/index.php/joiv/article/view/3102]
- 62. Salp Swarm Algorithm Optimized A* Algorithm and ... [https://www.mdpi.com/2076-3417/15/10/5583]
- 63. Improved Whale Optimization Algorithm for Increasing the ... [https://www.sciencedirect.com/science/article/abs/pii/S0094576525005727]
- 64. Active learning of neural population dynamics using two ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12466919/]
- 65. Resource-Efficient Decoding of Topological Color Codes ... [https://www.mdpi.com/2076-3417/15/16/8937]
- 66. A software platform for real-time and adaptive ... [https://www.nature.com/articles/s41467-025-64856-3]
- 67. Dynamical flexible inference of nonlinear latent factors and ... [https://www.nature.com/articles/s41551-023-01106-1]
- 68. Artificial intelligence in drug discovery and development - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC7577280/]
- 69. Advances in Zeroing Neural Networks: Convergence ... [https://www.mdpi.com/2227-7390/13/11/1801]
- 70. Flexible neural population dynamics govern the speed and ... [https://www.nature.com/articles/s41467-024-50563-y]
- 71. Population-Based Search Method Using Uncertainty ... [https://arxiv.org/html/2510.16386v1]